Phytochemical profiling and evaluation of antioxidant and antibacterial activities of Catharanthus roseus ethanol extract
*Article not assigned to an issue yet
Research Articles | Published: 03 December, 2025
First Page: 0
Last Page: 0
Views: 77
Keywords: Catharanthus roseus, GC–MS profiling, Fatty acid esters, Antioxidant activity, Antibacterial activity
Abstract
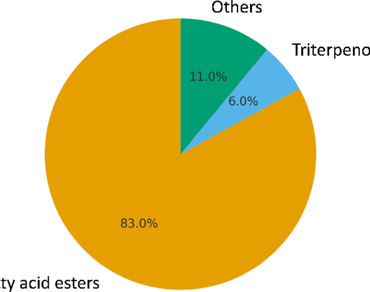
Catharanthus roseus is a medicinal plant rich in secondary metabolites with diverse pharmacological activities. This study investigated the phytochemical composition, antioxidant, and antibacterial activities of the ethanol extract of C. roseus. Phytochemical screening confirmed the presence of phenolics, flavonoids, and terpenoids, with total phenolic and flavonoid contents of 45.23 mg gallic acid equivalents (GAE)/g and 37.43 mg rutin equivalents (RE)/g extract, respectively. Gas chromatography–mass spectrometry (GC–MS) profiling identified 17 major compounds, dominated by fatty acid esters (83% of total peak area), particularly polyunsaturated fatty acid esters such as 8,11,14-docosatrienoic acid methyl ester (19.57%) and 9,12,15-octadecatrienoic acid ethyl ester (15.22%). Triterpenoids represented 6.15%, while benzenoid derivatives, amides, and hydrocarbons comprised ~ 11%. The ethanol extract exhibited moderate antioxidant activity with a half maximal inhibitory concentration (IC₅₀) of 245.65 µg/mL in the 2,2-diphenyl-1-picrylhydrazyl (DPPH) assay, compared to 12.71 µg/mL for vitamin C. It also showed dose-dependent antibacterial activity, with Escherichia coli being the most sensitive strain (8.00 mm at 50 mg/mL) and Salmonella sp. the least (2.67 mm). Correlation analysis revealed that antioxidant activity was primarily driven by fatty acid esters, whereas antibacterial effects resulted from a synergistic contribution of both fatty acid esters and triterpenoids. These findings support the traditional use of C. roseus and highlight its potential as a source of natural antioxidant and antimicrobial agents.
References
Ayoub Z, Mehta A, Mishra S (2017) Medicinal plants as natural antioxidants: a review. J Bot Soc 48:1–16
Baccouch R, Shi Y, Vernay E et al (2023) The impact of lipid polyunsaturation on the physical and mechanical properties of lipid membranes. Biochimica Et Biophysica Acta (BBA) 1865:184084. https://doi.org/10.1016/j.bbamem.2022.184084
Chakraborty S, Majumder S, Ghosh A, Saha S, Bhattacharya M (2021) Metabolomics of potential contenders conferring antioxidant property to varied polar and non-polar solvent extracts of Edgaria darjeelingensis C.B.Clarke. Bull Natl Res Cent 45:48. https://doi.org/10.1186/s42269-021-00503-3
Chandimali N, Bak SG, Park EH et al (2025) Free radicals and their impact on health and antioxidant defenses: a review. Cell Death Discov 11:19. https://doi.org/10.1038/s41420-024-02278-8
Chauhan N, Khan A, Farooq U (2023) Synergistic effect of combined antibiotic and methanolic extracts of Withania somnifera and Catharanthus roseus against MDR Salmonella enterica serovar Typhi. Adv Gut Microbiome Res 2023:8836886. https://doi.org/10.1155/2023/8836886
Chen Y, Qiu X, Yang J (2021) Comparing the in vitro antitumor, antioxidant and anti-inflammatory activities between two new very long chain polyunsaturated fatty acids, docosadienoic acid (DDA) and docosatrienoic acid (DTA), and docosahexaenoic acid (DHA). Nutr Cancer 73:1697–1707. https://doi.org/10.1080/01635581.2020.1804949
Dhankhar S, Dhankhar S, Ruhil S, Balhara M, Malik V, Chhillar A (2014) Isolation and biological evaluation of novel tetracosahexaene hexamethyl, an acyclic triterpenoids derivatives and antioxidant from Justicia adhatoda. Comb Chem High Throughput Screen. https://doi.org/10.2174/1386207317666140708091552
Dikamu M, Ezez D, Mamo A, Tefera M (2025) Analysis of phytochemical constituents using GC–MS, evaluation of antioxidants and antibacterial activities of Maerua oblongifolia root bark extracts. Discov Appl Sci 7:417. https://doi.org/10.1007/s42452-025-06939-w
El-Beltagi HS, El-Sayed SM, Abdelhamid AN et al (2023) Potentiating biosynthesis of alkaloids and polyphenolic substances in Catharanthus roseus plant using ĸ-carrageenan. Molecules 28(8):3642. https://doi.org/10.3390/molecules28083642
Espejel-Nava JA, Vega-Avila E, Alarcon-Aguilar F et al (2018) A phenolic fraction from Catharanthus roseus L. stems decreases glycemia and stimulates insulin secretion. Evid Based Complement Altern Med 2018:7191035. https://doi.org/10.1155/2018/7191035
Faure L, Cavazos R, Khan BR et al (2015) Effects of synthetic alkamides on Arabidopsis fatty acid amide hydrolase activity and plant development. Phytochemistry 110:58–71. https://doi.org/10.1016/j.phytochem.2014.11.011
Ferreres F, Pereira DM, Valentão P, Andrade PB, Seabra RM, Sottomayor M (2008) New phenolic compounds and antioxidant potential of Catharanthus roseus. J Agric Food Chem 56:9967–9974. https://doi.org/10.1021/jf8022723
Govindasamy C, Srinivasan R (2012) In vitro antibacterial activity and phytochemical analysis of Catharanthus roseus (Linn.) G. Don. Asian Pac J Trop Biomed 2:S155–S158. https://doi.org/10.1016/S2221-1691(12)60148-8
Gupta V, Tyagi S, Tripathi R (2023) Hexadecanoic acid methyl ester, a potent hepatoprotective compound in leaves of Pistia stratiotes L. Appl Biol Chem J 4(4):118–120. https://doi.org/10.52679/tabcj.2023.0012
Harborne AJ (1998) Phytochemical methods: a guide to modern techniques of plant analysis. Springer, Dordrecht
Herndon JL, Peters RE, Hofer RN, Simmons TB, Symes SJ, Giles DK (2020) Exogenous polyunsaturated fatty acids (PUFAs) promote changes in growth, phospholipid composition, membrane permeability and virulence phenotypes in Escherichia coli. BMC Microbiol 20:305. https://doi.org/10.1186/s12866-020-01988-0
Jannus F, Sainz J, Reyes-Zurita FJ (2024) Principal bioactive properties of oleanolic acid, its derivatives, and analogues. Molecules 29(14):3291. https://doi.org/10.3390/molecules29143291
Javed MR, Salman M, Tariq A et al (2022) The antibacterial and larvicidal potential of bis-(2-ethylhexyl) phthalate from Lactiplantibacillus plantarum. Molecules 27(21):7220. https://doi.org/10.3390/molecules27217220
Konappa N, Udayashankar AC, Krishnamurthy S, Pradeep CK, Chowdappa S, Jogaiah S (2020) GC–MS analysis of phytoconstituents from Amomum nilgiricum and molecular docking interactions of bioactive serverogenin acetate with target proteins. Sci Rep 10:16438. https://doi.org/10.1038/s41598-020-73442-0
Manurung H, Aryani R, Nugroho R, Sari Y, Chernovita R, Auliana (2019) Phytochemical analysis and antioxidant activity of leaves extracts of endemic plant. Int J Sci Technol Res 8:308–313
Mardani-Nejad S, Khavari-Nejad RA, Saadatmand S, Najafi F, Aberoomand Azar P (2016) Potent antioxidant properties of rolB-transformed Catharanthus roseus (L.) G. Don. Iran J Pharm Res 15:537–550
Mendonce KC, Palani N, Rajadesingu S, Radhakrishnan K, Ayyar M, Priya LS (2025) Pharmacological potential of bioactive compounds in Catharanthus roseus extract: a comprehensive review. Toxicol Rep 14:101998. https://doi.org/10.1016/j.toxrep.2025.101998
Mishra D, Chitara M, Chaturvedi P (2022) Study of phytochemicals, antioxidant activity and antimicrobial properties of Catharanthus roseus (L.) G. Don. Emerg Life Sci Res 8:75–79. https://doi.org/10.31783/elsr.2022.817579
Mokoroane KT, Karuppiah Pillai M, Magama S (2020) 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging activity of extracts from Aloiampelos striatula. Food Res 4:2062–2066. https://doi.org/10.26656/fr.2017.4(6).241
Nakaziba R, Amanya SB, Sesaazi CD, Byarugaba F, Ogwal-Okeng J, Alele PE (2022) Antimicrobial bioactivity and GC-MS analysis of different extracts of Corchorus olitorius L leaves. Sci World J 2022:3382302. https://doi.org/10.1155/2022/3382302
Nisa S, Bibi Y, Masood S et al (2022) Isolation, characterization and anticancer activity of two bioactive compounds from Arisaema flavum (Forssk.) Schott. Molecules 27(22):7932. https://doi.org/10.3390/molecules27227932
Ng SCW, Furman R, Axelsen PH, Shchepinov MS (2022) Free radical chain reactions and polyunsaturated fatty acids in brain lipids. ACS Omega 7:25337–25345. https://doi.org/10.1021/acsomega.2c02285
Pandya D, Patel M, Patel J, Dabgar Y (2024) Antibacterial activity of methanolic and ethanolic extracts of leaves of Catharanthes roseus L. J Med Plants Stud 12:135–138. https://doi.org/10.22271/plants.2024.v12.i1b.1635
Radi MH, El-Shiekh RA, El-Halawany AM, Abdel-Sattar E (2023) Friedelin and 3β-friedelinol: pharmacological activities. Rev Bras Farmacogn 33:886–900. https://doi.org/10.1007/s43450-023-00415-5
Rani J, Kapoor M, Dhull SB, Gökşen G, Jurić S (2023) Identification and assessment of therapeutic phytoconstituents of Catharanthus roseus through GC-MS analysis. Separations 10:340. https://doi.org/10.3390/separations10060340
Romero LO, Massey AE, Mata-Daboin AD et al (2019) Dietary fatty acids fine-tune Piezo1 mechanical response. Nat Commun 10:1200. https://doi.org/10.1038/s41467-019-09055-7
Sá S, Chaul LT, Alves VF et al (2018) Phytochemistry and antimicrobial activity of Campomanesia adamantium. Rev Bras Farmacogn 28:303–311. https://doi.org/10.1016/j.bjp.2018.02.008
Saquib SA, AlQahtani NA, Ahmad I, Kader MA, Al Shahrani SS, Asiri EA (2019) Evaluation and comparison of antibacterial efficacy of herbal extracts in combination with antibiotics on periodontal pathobionts: an in vitro microbiological study. Antibiotics 8(3):89. https://doi.org/10.3390/antibiotics8030089
Saravanakumar K, Chelliah R, Ramakrishnan SR, Kathiresan K, Oh D-H, Wang M-H (2018) Antibacterial and antioxidant potentials of non-cytotoxic extract of Trichoderma atroviride. Microb Pathog 115:338–342. https://doi.org/10.1016/j.micpath.2017.12.081
Sarkar BK, Polash MJI, Islam MJ et al (2025) Unlocking the therapeutic potential of Catharanthus roseus leaves via in-vitro, in-vivo, and in-silico study. Sci Rep 15:25909. https://doi.org/10.1038/s41598-025-96643-x
Shaaban MT, Ghaly MF, Fahmi SM (2021) Antibacterial activities of hexadecanoic acid methyl ester and green-synthesized silver nanoparticles against multidrug-resistant bacteria. J Basic Microbiol 61:557–568. https://doi.org/10.1002/jobm.202100061
Shahidi F, Zhong Y (2015) Measurement of antioxidant activity. J Funct Foods 18:757–781. https://doi.org/10.1016/j.jff.2015.01.047
Shil A, Mukherjee S, Biswas P et al (2023) Catharanthus roseus (L.) G. Don counteracts the ampicillin resistance in multiple antibiotic-resistant Staphylococcus aureus by downregulation of PBP2a synthesis. Biol. https://doi.org/10.1515/biol-2022-0718
Tolba S, Mohammed H, Ghareeb M, Mohamed A (2024) Antidiabetic activity and GC-MS analysis of n-hexane leaf extract of Codiaeum variegatum (Euphorbiaceae). Azhar Int J Pharm Med Sci. https://doi.org/10.21608/aijpms.2024.264259.1250
Uzma F, Chowdappa S, Roy A et al (2024) GC-MS-guided antimicrobial defense responsive secondary metabolites from the endophytic Fusarium solani isolated from Tinospora cordifolia and their multifaceted biological properties. Appl Biochem Biotechnol 196:3010–3033. https://doi.org/10.1007/s12010-023-04669-6
Velu G, Mary SA (2013) GC-MS analysis of petroleum ether and ethanol leaf extracts from Abrus precatorius Linn. Int J Pharm Bio Sci 4:P37–P44
Verstraeten S, Catteau L, Boukricha L, Quetin-Leclercq J, Mingeot-Leclercq M-P (2021) Effect of ursolic and oleanolic acids on lipid membranes: studies on MRSA and models of membranes. Antibiotics 10(11):1381. https://doi.org/10.3390/antibiotics10111381
Yadav A, Yadav M, Kumar S, Sharma D, Yadav JP (2018) In vitro antioxidant activities and GC-MS analysis of different solvent extracts of Acacia nilotica leaves. Indian J Pharm Sci 80:892. https://doi.org/10.4172/pharmaceutical-sciences.1000436
Yu H, Chen Y, Cheng Z et al (2023) Anti-inflammatory oleanane-type triterpenoids produced by Nonomuraea sp. MYH522 through microbial transformation. J Agric Food Chem 71:3777–3789. https://doi.org/10.1021/acs.jafc.2c09062
Zhu J, Wang M, Wen W, Yu R (2015) Biosynthesis and regulation of terpenoid indole alkaloids in Catharanthus roseus. Pharmacogn Rev 9:24–28. https://doi.org/10.4103/0973-7847.156323
Zouine N, Ghachtouli NE, Abed SE, Koraichi SI (2024) A comprehensive review on medicinal plant extracts as antibacterial agents: factors, mechanism insights and future prospects. Sci Afr 26:e02395. https://doi.org/10.1016/j.sciaf.2024.e02395
Author Information
An Giang University, Long Xuyen ward, Vietnam