Phytochemical profiling, antioxidant and antibacterial activities of phenolic extracts from Smilax aspera and Atractylis humilis roots
*Article not assigned to an issue yet
Zakhrouf Zohra, Harrat Mohamed, Elhouiti Fatiha, Válega Mónica, Yousfi Mohamed, Boudjeniba Messaoud
Research Articles | Published: 26 November, 2025
First Page: 0
Last Page: 0
Views: 72
Keywords: n Smilax asperan , n Atractylis humilisn , Antioxidant and antimicrobial activities
Abstract
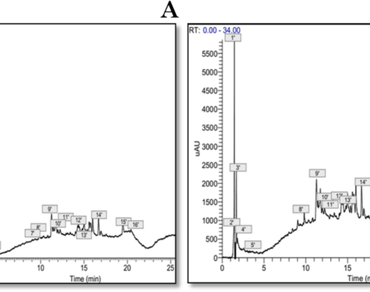
This study evaluates the chemical composition, the antioxidant and antimicrobial activities of phenolic extracts of Smilax aspera and Atractylis humilis roots from Algeria. The total phenolic, the flavonoid and the condensed tannin amounts were analyzed in different extracts using Folin-Ciocalteu, aluminum chloride and vanillin assays, respectively. The UHPLC-DAD-ESI-MSn analysis was used for the qualitative elucidation of bioactive compounds. The in vitro antioxidant capacity of extracts was evaluated by DPPH, iron chelating ABTS· + and FRAP assays. The different extracts were tested against five bacterial strains (Escherichia coli, Pseudomonas aeruginosa, Yersinia enterocolitica, Klebsiella pneumoniae and Staphylococcus aureus.) and one fungal strain (Candida albicans). Among the analyzed Smilax aspera and Atractylis humilis polar extracts, the ethyl acetate fraction of the S. aspera hydro-methanolic system had the highest total phenolic and flavonoid contents. UHPLC-DAD-ESI-MSn analysis revealed glycoside saponins in S. aspera and various hydroxycinnamic acid derivatives in A. humilis extracts. The ethyl acetate fraction of S. aspera hydromethanolic system extract demonstrated strong antioxidant activity (IC50 = 54.12 ± 0.74 µg/mL and 14.65 ± 0.34 µg/mL in DPPH and ABTS assays, respectively), while the dichlomethane fraction demonstrated significantly high antioxidant activity (IC50 = 43.6 ± 0.71 μg/mL in FRAP assay). In addition, a moderate antibacterial effect was observed against Pseudomonas aeruginosa, Klebsiella pneumoniae, and Staphylococcus aureus, as well as a remarkable efficiency against Candida albicans. According to the findings, the roots of the two plants contain a variety of phytochemicals with significant antibacterial and antioxidant properties, which support their use in traditional medicine.
References
Ahmad,V.U., and Basha, A. (2006) Spectroscopic Data of Steroid Glycosides: Stigmastanes, Furostanes, Spirtostanes Volume 2. Springer Science+Business Media, LLC
Akula R, Ravishankar GA (2011) Influence of abiotic stress signals on secondary metabolites in plants. Plant Signal Behav 6(11):1720–1731. https://doi.org/10.4161/psb.6.11.17613
Amadoa PA, Ferrazb V, da Silvac DB, Carolloc CA, Castroa AHF, dos Santos Lima LAR (2018) Chemical composition, antioxidant and cytotoxic activities of extracts from the leaves of Smilax brasiliensis Sprengel (Smilacaceae). Nat Prod Res 32(5):610–615. https://doi.org/10.1080/14786419.2017.1327861
Bader G, Seibold M, Tintelnot K, Hiller K (2000) Cytotoxicity of triterpenoid saponins. Part 2: Relationships between the structures of glycosides of polygalacic acid and their activities against pathogenic Candida species. Pharmazie 55:72–74
Belhouchet Z, Sautour M, Miyamoto T, Lacaille-dubois MA (2008) Steroidal Saponins from the Roots of Smilax aspera subsp. Mauritanica Chem Pharm Bull 56(9):1324–2132
Benzie IFF, Strain JJ (1996) The Ferric Reducing Ability of Plasma (FRAP) as a Measure of ‘“Antioxidant Power”’: The FRAP Assay. Anal Biochem 239:70–76
Boudebaz, K., Nia, S., Trabelsi-Ayadi, M., and Cherif J. K. (2015): The effect of extraction method on antioxidant activity of Atractylis babelii Hochr. leaves and flowers extracts. Algerian Journal of Natural Products 3:2 . 146–152
Brand-Williams W, Cuvelier ME, Berset C (1995) Use of a free radical method to evaluate antioxidant activity. LWT 28:25–30
Broadhurst RB, Jones WT (1978) Analysis of Condensed Tannins Using Acidified Vanillin. J Sci Fd Agric 29:788–794
Bystroma, L.M., Lewisa, B.A., Brownb, D.L., Rodriguezc, E., and L.Obendorf,R. (2008): Characterization of phenolics by LC-UV/vis, LC-MS/MS and sugars by GC in Melicoccus bijugatus Jacq. ‘Montgomery’ fruits. Food Chem. 111(4): 1017–1024. https://doi.org/10.1016/j.foodchem.2008.04.058
Carius B, Silva H, Silva AMS, Pinto DCGA (2022) Chemical profiling of Limonium vulgare Mill. using UHPLC-DAD-ESI/MS2 and GC-MS analysis. Appl Sci 12:6384. https://doi.org/10.3390/app12136384
Chabani S, Haba H, Lavaud C, Benkhaled M, Harakat D (2013) Flavonoid glycosides and triterpenoids from Atractylis flava. Phytochem Lett 6:9–13. https://doi.org/10.1016/j.phytol.2012.10.004
Chabani S, Lavaud C, Benkhaled M, Harakat D, Long C, Haba H (2016) Three new oleanane-type triterpene saponins from Atractylis flava. Phytochem Lett 15:88–93. https://doi.org/10.1016/j.phytol.2015.11.017
Challinor VL, Parsons PG, Chap S, White EF, Blanchfield JT, Lehmann RP, De Voss JJ (2012) Steroidal saponins from the roots of Smilax sp.: structure and bioactivity. Steroids 77:504–511. https://doi.org/10.1016/j.steroids.2012.01.009
Chen FJ, Long XH, Liu ZP, Shao HB, Liu L (2014) Analysis of phenolic acids of Jerusalem artichoke (Helianthus tuberosus L.) responding to salt-stress by liquid chromatography/tandem mass spectrometry. Sci World J. https://doi.org/10.1155/2014/568043
Chen, L., Yin, H., Lan, Z., i Ma, S., Zhang, C., Yang, Z., Li, P., Lin, B. (2011) :Anti-hyperuricemic and nephroprotective effects of Smilax china L. Journal of Ethnopharmacology 135, 399–405 https://doi.org/10.1016/j.jep.2011.03.033.
Davis, C. C and Choisy, P.(2023): Medicinal plants meet modern biodiversity.Current Biology, Volume 34, Issue 4, R158 - R17
Dewanto V, Wu XZ, Adom KK, Liu RH (2002) Therma, processing enhances the nutritional value of tomatoes by increasing total antioxidant activity. J Agric Food Chem 50:3010–3014
El Rhaffari, L.Z. (2002): La pratique de la phytothérapie dans le sud-est du Maroc (Tafilalet). Un savoir empirique pour une pharmacopée rénovée. In: Dans, J., Fleurentin (Éd.) Des sources du savoir aux médicaments du futur. IRD editions, Paris, 293–318
El Sayed AM, Basam SM, El-Naggar EBA, Marzouk HS, El-Hawary S (2020) LC–MS/MS and GC–MS profiling as well as the antimicrobial effect of leaves of selected Yucca species introduced to Egypt. Sci Rep 10:17778. https://doi.org/10.1038/s41598-020-74440-y
Escobar-Avello D, Lozano-Castellón J, Mardones C, Pérez AJ, Saéz V, Riquelme S, Baer DV, Vallverdú-Queralt A (2019) Phenolic profile of grape canes: novel compounds identified by LC-ESI-LTQ-Orbitrap-MS. Molecules 24:3763. https://doi.org/10.3390/molecules24203763
Fidrianny I, Anggraeni NAS, Insanu M (2018) Antioxidant properties of peels extracts from three varieties of banana (Musa sp.) grown in West Java-Indonesia. Int Food Res J 25(1):57–64
Fonseca JC, Barbosa MA, Silva ICA, Duarte-Almeida JM, Castro AHF, dos Santos Lima LAR (2017) Antioxidant and allelopathic activities of Smilax brasiliensis Sprengel (Smilacaceae). S Afr J Bot 111:336–340. https://doi.org/10.1016/j.sajb.2017.04.003
Fukumoto LR, Mazza G (2000) Assessing antioxidant and prooxidant activities of phenolic compounds. J Agric Food Chem 48:3597–3604
Gouveia S, Castilho PC (2011) Characterization of phenolic acid derivatives and flavonoids from different morphological parts of Helichrysum obconicum by a RP-HPLC–DAD–ESI-MSn method. Food Chem 129:333–344. https://doi.org/10.1016/j.foodchem.2011.04.078
Hachani S, Hamia C, Boukhalkhal S, Silva AMS, Djeridane A, Yousfi M (2018) Morphological, physico-chemical characteristics and effects of extraction solvents on UHPLC-DAD-ESI-MSn profiling of phenolic contents and antioxidant activities of five date cultivars (Phoenix dactylifera L.) growing in Algeria. NFS J 13:10–22. https://doi.org/10.1016/j.nfs.2018.10.001
Hatano T, Kagawa H, Yasuhara T, Okuda T (1988) Two new flavonoids and other constituents in licorice root: their relative astringency and radical scavenging effects. Chem Pharm Bull 36:2090–2097
Kakouri E, Hatziagapiou K, Kanakis C, Nikola O, Lambrou GI, Trigas P, Kanaka-Gantenbein C, Tarantilis PA (2023) Cytotoxic and antioxidant activity of a chemically characterized extract of Smilax aspera leaves and stems. Appl Sci 13:4784. https://doi.org/10.3390/app13084784
Kim JS, Lee JH (2020) Correlation between solid content and antioxidant activities in Umbelliferae salad plants. Prev Nutr Food Sci 25(1):84–92. https://doi.org/10.3746/pnf.2020.25.1.84
Kozłowska M, Scibisz I, Przybył JL, Laudy AE, Majewska E, Tarnowska K, Małajowicz J, Ziarno M (2022) Antioxidant and Antibacterial Activity of Extracts from Selected Plant Material. Appl Sci 12:9871. https://doi.org/10.3390/app12199871
Lachachi-Allal, A. (2021) :Caractérisations chimiques, activités biologiques et valorisation d’extraits de plantes sous-utilisées de la région de l’ouest d’Algérie. Phd thesis
Mahcene S, Elhouiti F, Mennai I, Pinto DCGA, Tahri D, Ouinten M, Yousfi M (2023) Chemical composition, antimicrobial, antiparasitic, and cytotoxic activities of Rhanterium intermedium Pomel leaves essential oil. Arab J Sci Eng. https://doi.org/10.1007/s13369-023-07913-7
Melakhessou MA, Benkiki N, Marref SE, Bouzidi S (2018) Anti-inflammatory, anti-pyretic and acute toxicity effects of n-butanol extract of Atractylis flava Desf in rats. Pharmacogn J 10(4):763–767
Miller NJ, Diplock AT, Rice-Evans CA (1995) Evaluation of the total antioxidant activity as a marker of the deterioration of apple juice on storagej. Agric Food Chem 43:1794–1801
Moghaddam, Z. P., Mohammadi, A., alesheikh, P., Feyzi, P., Haghbin, A., Mollazadeh, S., Sabeti, Z., Nakhlband, A., Kasaian, J. (2021). Antibacterial, Antifungal, and Antioxidant Activity of Cleome coluteoides: An In Vitro Comparative Study Between Leaves, Stems, and Flowers. Turkish Journal of Pharmaceutical Sciences, 18(1), 10–16. http/ https://doi.org/10.4274/tjps.galenos.2019.59320
Moraisa, M.I., Pintoa, M.E.A., Arau´joa, S.G., Castroa, A. F., Duarte-Almeidaa J.M., Rosab, L.H., Rosab, C.A., Johannb, S., and dos Santos Lima, L.A.R. (2014) Antioxidant and antifungal activities of Smilax campestris Griseb. (Smilacaceae). atural Product Research https://doi.org/10.1080/14786419.2014.895728
Rajesh V, Peruma P (2013) In vivo assessment of antidiabetic and antioxidant activities of methanol extract of Smilax zeylanica leaves in wistar rats. Orient Pharm Exp Med. https://doi.org/10.1007/s13596-013-0137-z
Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-evans C (1999) Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med 26(Nos. 9/10):1231–1237
Rudrapal, M.; Rakshit, G.; Singh, R.P.; Garse, S.; Khan, J.; Chakraborty, S. Dietary Polyphenols. (2024): Review on Chemistry/Sources, Bioavailability/Metabolism, Antioxidant Effects, and Their Role in Disease Management. Antioxidants 2024, 13, 429. https://doi.org/10.3390/antiox13040429
Sarkar, A., Ghosh, P., Poddar, P., Sarkar, T., Choudhury, S., and Chatterjee,S. (2020) : Phytochemical, botanical and Ethnopharmacological study of Scoparia dulcis Linn. (Scrophulariaceae): A concise review. The Pharma Innovation Journal; 9(7): 30–35. https://doi.org/10.22271/tpi.2020.v9.i7a.5049
Sautour M, Miyamoto T, Lacaille-Dubois M-A (2006) Bioactive steroidal saponin from Smilax medica. Planta Med 72:667–670
Shu X-S, Gao Z-H, Yang X-L (2006) Anti-inflammatory and anti-nociceptive activities of Smilax china L. aqueous extract. J Ethnopharmacol 103:327–332. https://doi.org/10.1016/j.jep.2005.08.004
Sifouane, S. (2021) Etude phytochimique de deux plantes Atractylis humilis et Carduncellus pinnatus (Asteraceae). Phd thesis.
Sifouane S, Benabdelaziza I, Benkhaleda M, Gómez-Ruizb S, Carralerob S, Haba H (2020) A new aryltetralin lignan and other phytoconstituents from Atractylis humilis. Biochem Syst Ecol 90:104018. https://doi.org/10.1016/j.bse.2020.104018
Singleton V, Orthofer R, Lamuela-Ravent RM (1999) Analysis of total phenols and other oxidation subsrates and antioxidants by means of Folin-Ciocalteu Reagent. Methods Enzymol 299:152–178
Sivapriya M, Dinesha R, Harsha R, Gowda SST, Srinivas L (2011) Antibacterial activity of different extracts of Sundakai (Solanum torvum) fruit coat. Int J Biol Chem 5:61–67
Sobolewska D, Michalska K, Podolak I, Grabowska K (2016) Steroidal saponins from the genus Allium. Phytochem Rev 15:1–35. https://doi.org/10.1007/s11101014-9381-1
Stojković,D., Petrović, J., Soković, M., Glamočlijaa, J., KukićMarković, J., Petrović, S. (2013): In situ antioxidant and antimicrobial activities of naturally occurring caffeic acid, p-coumaric acid and rutin, using food systems. journal of the Science of Food and Agriculture, 93(13), 3205–3208. https://doi.org/10.1002/jsfa.6156
Teanpaisan R, Kawsud P, Pahumunto N, Puripattanavong J (2017) Screening for antibacterial and antibiofilm activity in Thai medicinal plant extracts against oral microorganisms. J Tradit Complement Med 7:172–177
Torres-Benítez A, Ortega-Valencia JE, Sanchez M, Divakar PK, Simirgiotis MJ, Gómez-Serranillos MP (2022) Metabolomic profiling, antioxidant and enzyme inhibition properties and molecular docking analysis of Antarctic lichens. Molecules 27:8086. https://doi.org/10.3390/molecules27228086
Wiegand I, Hilpert K, Hancock REW (2008) Agar and broth dilution methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substances. Nat Protoc. https://doi.org/10.1038/nprot.2007.521
Xu, Z., Chang, L. (2017). Asteraceae. In: Identification and Control of Common Weeds: Volume 3. Springer, Singapore. https://doi.org/10.1007/978-981-10-5403-7_20
Yuzuak, S., Ballington, J., and Xie, D.U. (2018). HPLC-qTOF-MS/MS-Based Profiling of Flavan-3-ols and Dimeric Proanthocyanidins in Berries of Two Muscadine Grape Hybrids FLH 13–11 and FLH 17–66. Metabolites, 8, 57; https://doi.org/10.3390/metabo8040057
Zhang YJ, Gan R-Y, Li S, Zhou Y, Li AN, Xu DP, Li HB (2015) Antioxidant Phytochemicals for the Prevention and Treatment of Chronic Diseases. Molecules 20:21138–22115. https://doi.org/10.3390/molecules201219753
Zhishen J, Mengcheng T, Jiangming W (1999) The determination of flavonoid contents in mulberry and their scavenging effect on superoxide radicals. Food Chem 64:555–559
Zubair M, Rizwan K, Rashid U, Saeed R, Saeed AA, Rasool N, Riaz M (2013) GC/MS profiling, in vitro antioxidant, antimicrobial and haemolytic activities of Smilax macrophylla leaves. Arab J Chem. https://doi.org/10.1016/j.arabjc.2013.04.024
Tian LW, Zhang Z, Long HL, Zhang YJ. (2017) Steroidal Saponins from the Genus Smilax and Their Biological Activities. Nat Prod Bioprospect. 7(4):283–298. https://doi.org/10.1007/s13659-017-0139-5
Author Information
Laboratoire des Sciences Fondamentales, University of Amar Telidji / Laghouat, Laghouat, Algeria