Plant beneficial effects of Trichoderma spp. suppressing Fusarium wilt and enhancing growth in Tomato
Kumar Krishna, Thakur Parika, Rathore Utkarsh Singh, Kumar Sandeep, Mishra R. K., Amaresan N., Pandey Sonika, Mishra Monika
Research Articles | Published: 31 July, 2021
First Page: 188
Last Page: 195
Views: 4067
Keywords: Trichoderma asperellum , Bioinoculant, Biotic stress, Fusarium wilt, Vigour index
Abstract
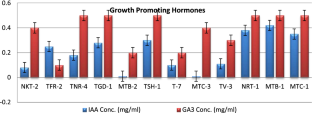
Trichoderma spp. are widely used as beneficial fungi for disease management, plant growth promotion, induced resistance, and abiotic stress management. Henceforth, plays a pivotal role in sustainable agriculture around the world. The study aimed to identify a promising biocontrol agent with multi-trait properties wherein isolates of Trichoderma spp. were identified on the basis of their morphological and molecular characteristics for species-level determination. Twelve Trichoderma isolates tested in-vitro against Fusarium solani and their biochemical responses concerning defense enzymes, growth enzymes, and lytic enzymes were also assessed, further validated under pot culture conditions. Under thein-vitro study, the highest percent inhibition, specific activity of defense enzymes (PO, PPO, and SOD) and lytic enzymes (cellulase, Xylanase, CMCase, and Chitinase) production were recorded with T. asperellum (TGD-I and TSH-I) and T. harzianum (MTB-I). These three isolates exhibited growth hormones ranging from 0.01 to 0.42 μl/ml (IAA) and 0.2–0.5 μl/ml (GA3) and also showed the highest root and shoot growth promotion. This study proves that T. asperellum and T. harzianum initially isolated from hot humid tropical climate may be used as bio inoculants in subtropical areas of India for biotic stress management and plant growth promotion in vegetable crops.
References
Agrios GN (2005) Plant pathology, 5th edn. Academic Press, London
Ahmad I, Bhagat S, Sharma TVRS, Kumar K, Simachalam P, Srivastava RC (2010) ISSR and RAPD marker based DNA fingerprinting and diversity assessment of Annona spp. South Andamans Indian J Hort 67(2):147–151
Akinnifesi TA, Asubiojo OI, Amusan AA (2006) Effects of fungicide residues on the Physio-chemical characteristics of soils of a major cocoa-producing area of Nigeria. Sci Total Environ 366(2–3):876
Benitez T, Rincon AM, Limon MC, Codon AC (2004) Biocontrol mechanisms of Trichoderma strains. Int Microbiol 7:249–260
Chen XH, Koumoutsi A, Scholz R, Eisenreich A, Schneider K, Heinemeyer I, Morgenstern B, Voss B, Hess WR, Reva O, Junge H, Voigt B, Jungblut PR, Vater J, Sussmuth R, Liesegang H, Strittmatter A, Gottschalk G, Borriss R (2007) Comparative analysis of the complete genome sequence of the plant growth-promoting bacterium Bacillus amyloliquefaciens FZB42. Nat Biotechnol 25(9):1007–1014
Deep S, Sharma P, Behera N (2014) Optimization of extracellular cellulase enzyme production from Alternaria brassicicola. Int J Curr Microbiol App Sci 3(9):127–139
Elad Y, Kapat A (1999) The role of Trichoderma harzianum protease in the biocontrol of Botrytis cinerea. Eur J Plant Pathol 105:177–189
El-Sobky MA, Fahmi AI, Eissa RA, El-Zanaty AM (2019) Genetic characterization of Trichoderma spp. isolated from different locations of Menoufia, Egypt, and assessment of their antagonistic ability. J Microb Biochem Technol 11:1
FAOSTAT (2019) http://www.fao.org/faostat/en/#data/QC
Ghanshyam C, Jain M (2009) Role of auxin-responsive genes in biotic stress responses. Plant Signal Behav 4:846–848
Gomes EV, Ulhoa CJ, Cardoza RE, Silva RN, Gutiérrez S (2017) Involvement of Trichoderma harzianum Epl-1 protein in the regulation of botrytis virulenceand tomato defense-related genes. Front Plant Sci 8:880
Gravel V, Antoun H, Tweddel RJ (2007) Growth stimulation and fruit yield improvement of greenhouse tomato plants by inoculation with Pseudomonas putida or Trichoderma atroviride: possible role of indole acetic acid (IAA). Soil Biol Biochem 39:1968–1977
Guo Y, Ghirardo A, Weber B, Schnitzler JP, Benz JP, Rosenkranz M (2019) Trichoderma species differ in their volatile profiles and in antagonism toward Ectomycorrhiza Laccaria bicolor. Front Microbiol 10:891
Harman GE (2011) Multifunctional fungal plant symbionts: new tools to enhance plant growth and productivity. New Phytol 189:647–649
Jayalakshmi SK, Raju S, Usha Rani S, Benagi SK (2009) Trichoderma harzianum L1 as a potential source for lytic enzymes and elicitor of defense responses in chickpea (Cicer arietinum L.) against wilt disease caused by Fusarium oxysporum f. sp. ciceri. Aust J Crop Sci 3:44–52
Karima HHE, Gamal NG (2012) In vitro study on Fusarium solani and Rhizoctonia solani isolates causing the damping off and root rot diseases in tomatoes. Nat Sci 10(11):16–25
Kärkönen A, Warinowski T, Teeri TH, Simola LK, Fry SC (2009) On the mechanism of apoplastic H2O2 production during lignin formation and elicitation in cultured spruce cells—peroxidases after elicitation. Planta 230(3):553–567
Khatri DK, Tiwari DN, Bariya HS (2017) Chitinolytic efficacy and secretion of cell wall-degrading enzymes from Trichoderma spp. in response to Phytopathological fungi. J Appl Biol Biotechnol 5(6):1–8
Kumar K, Amaresan N, Bhagat S, Madhuri K, Udhayaraj P, Srivastava RC (2011) Genetic and physiological relatedness of antagonistic Trichoderma isolates against soil borne plant pathogenic fungi. Arch Phytopathol Plant Protect 44(14):1399–1409
Kumar K, Amaresan N, Bhagat S, Madhuri K, Srivastava RC (2012) Isolation and characterization of Trichoderma spp. for antagonistic activity against root rot and foliar pathogens. Indian J Microbiol 52(2):137–144
Laws H (1991) Handbook of pesticide toxicology, 1st edn. Academic Press, India
Lorito M (1998) Chitinolytic enzymes and their genes. In: Harman GE, Kubicek CP (Eds). Trichoderma and Gliocladium, Edition. Taylor and Francis, London 2: 73–99
Manigundan K, Sakthivel K, Gautam RK, Kumar K, Anantharaj A, Velmurugan A, Singh PK, Roy S, Dam (2016) Characterization of species from vegetable and spice rhizospheres of Andaman Islands for broad spectrum antagonism and plant growth promotion. J Environ Biol 341–347.
Mayer AM, Harel E (1979) Polyphenol oxidases in plants. Phytochemistry 18:193–214
Mishra RK, Monika M, Naimuddin KV (2018a) Trichoderma asperellum: a potential biocontrol agents against wilt of pigeonpea caused by Fusarium udum Butler. J Food Legumes 31:50–53
Mishra RK, Bohra A, Kamaal N, Kumar K, Sujayanand GK, Saabale PR, Naik SJ, Sarma BK, Kumar D, Mishra M, Srivastava DK, Singh NP (2018b) Utilization of biopesticides as sustainable solutions for management of pests in legume crops: achievements and prospects. Egyptian J Biol Pest Control. https://doi.org/10.1186/s41938-017-0004-1
Mishra RK, Pandey S, Mishra M, Rathore US, Naimuddin KK, Singh B (2020) Assessment of biocontrol potential of Trichoderma isolates against wilt in pulses. J Food Legumes 33:48–52
Mondejar RL, Ros M, Pascua JA (2011) Mycoparasitism-related genes expression of Trichoderma harzianum isolates to evaluate their efficacy as biological control agent. Biol Control 56:59–66
Murphy JF, Reddy MS, Ryu CM, Kloepper JW, Li R (2003) Rhizobacteria-mediated growth promotion of tomato leads to protection against Cucumber mosaic virus. Phytopathology 93:1301–1307
Raeder U, Broda P (1985) Rapid preparation of DNA from filamentous fungi. Lett Appl Microbiol 1:17–20
Ramezani H (2009) Efficacy of fungal and bacterial bioagents against Fusarium oxysporum f.sp. ciceri on chickpea. Plant Prot J 1:108–113
Shanmugaiah V, Balasubramanian N, Gomathinayagam S, Manoharan PT, Rajendran A (2009) Effect of single application of Trichoderma viride and Pseudomonas fluorescens on growth promotion in cotton plants. Afr J Agric Res 4(11):1220–1225
Author Information
Division of Crop Protection, Indian Institute of Pulses Research, Kanpur, India