Pollen characteristics and their implications for the conservation of Moroccan Cannabis cultivar
*Article not assigned to an issue yet
Research Articles | Published: 08 October, 2025
First Page: 0
Last Page: 0
Views: 2
Keywords: n Cannabis cultivation, Cultivars, Pollen germination, Pollen viability, Pollen production
Abstract
Pollen viability, germination rates, and abundance play a crucial role in the reproductive success and genetic integrity of Cannabis cultivars. This study aims to evaluate these traits in five cultivars (Amnesia nicon, Beldia, Coach + , Coach nicon, Critical + ) grown in Morocco, focusing on the local landrace ‘Beldia’. As Cannabis cultivation evolves under new legal and commercial pressures, traditional varieties face risks of genetic erosion. To assess male reproductive traits, in vitro tests were conducted using standardised procedures to ensure consistency across all samples. Results reveal significant variability among cultivars, with Beldia achieving the highest mean pollen viability (90.84%), reflecting its adaptation to local conditions. The lowest mean pollen viability was recorded for the introduced cultivar Coach + , which showed 66.04%. Germination rates showed notable differences, Amnesia nicon with 50.91% and Coach nicon, with 4.01%. Beldia and Coach + Cultivars underperformed compared to typical germination ranges, highlighting the influence of genetic and environmental factors on reproductive success. Pollen abundance also varied, with Critical + producing the highest pollen count (74.6 × 103 per anther), increasing the likelihood of cross-pollination with other cultivars. Further research is needed to examine how phenological stages, such as anthesis timing and cultivation periods, affect cross-pollination risk. This is crucial for developing strategies to preserve the genetic integrity and productivity of local cultivars, especially in the context of climate change. This study provides a foundation for breeding and conservation efforts to sustain the unique traits and yield potential of Moroccan Cannabis cultivars.
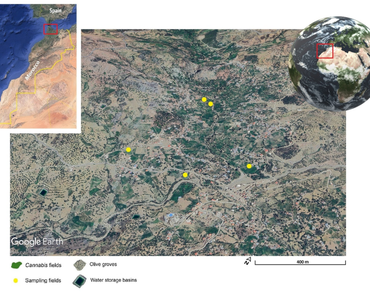
References
Abdelgadir HA, Johnson SD, Van Staden J (2012) Pollen viability, pollen germination and pollen tube growth in the biofuel seed crop Jatropha curcas (Euphorbiaceae). S Afr J Bot 79:132–139. https://doi.org/10.1016/j.sajb.2011.10.005
Ackerman JD (2000) Abiotic pollen and pollination: ecological, functional, and evolutionary perspectives. Pl Syst Evol 222:167–185. https://doi.org/10.1007/BF00984101
Amaducci S, Zatta A, Pelatti F, Venturi G (2008) Influence of agronomic factors on yield and quality of hemp (Cannabis sativa L.) fibre and implication for an innovative production system. Field Crops Res 107(2):161–169. https://doi.org/10.1016/j.fcr.2008.02.002
Araújo de Oliveira AC, da Silva LA, Polek M, Krueger R, Shepherd A, Volk GM (2021) Optimization of in vitro germination and cryopreservation conditions for preserving date palm pollen in the USDA national plant germplasm system. Plant Cell Tiss Organ Cult 144(1):223–232. https://doi.org/10.1007/s11240-020-01907-1
Bachir F, Eddouks M, Arahou M, Fekhaoui M (2022) Origin, early history, cultivation, and characteristics of the traditional varieties of Moroccan Cannabis sativa L. Cannabis Cannabinoid Res 7(5):603–615. https://doi.org/10.1089/can.2021.0020
Bellakhdar J (2013) L’histoire du chanvre au Maghreb. Hespéris-Tamuda 48:107–141
Bellakhdar J (2017) Les voies suivies par le chanvre dans sa conquête du Maghreb. HespérisTamuda LII(2):117–150
Benkirane C, Charif M, Müller CM, Taaifi Y, Mansouri F, Addi M, Bellaoui M, Serghini-Caid H, Elamrani A, Abid M (2024) Population structure and genetic diversity of Moroccan cannabis (Cannabis sativa L.) germplasm through simple sequence repeat (SSR) analysis. Genet Resour Crop Evol 71(5):2037–2051. https://doi.org/10.1007/s10722-023-01754-x
Bonini SA, Premoli M, Tambaro S, Kumar A, Maccarinelli G, Memo M, Mastinu A (2018) Cannabis sativa: a comprehensive ethnopharmacological review of a medicinal plant with a long history. J Ethnopharmacol 227:300–315. https://doi.org/10.1016/j.jep.2018.09.004
Brewbaker JL, Kwack BH (1964) The calcium ion and substances influencing pollen growth. North-Holland Publishing Company, Amsterdam
Brunet J, Ziobro R, Osvatic J, Clayton MK (2019) The effects of time, temperature and plant variety on pollen viability and its implications for gene flow risk. Plant Biol 21(4):715–722. https://doi.org/10.1111/plb.12959
Bulletin Officiel (2021) Dahir N◦ 1–21–59 Du 3 Hija 1442 (14 Juillet 2021) portant promulgation de La Loi N◦ 13–21 relative aux usages licites du Cannabis. http://www.sgg.gov.ma/BO/bo_fr/2021/BO_7022_Fr.pdf. Accessed 15 July 2024
Burke JJ, Velten J, Oliver MJ (2004) In vitro analysis of cotton pollen germination. Agron J 96(2):359–368. https://doi.org/10.2134/agronj2004.3590
Carrizo García C, Nepi M, Pacini E (2017) It is a matter of timing: asynchrony during pollen development and its consequences on pollen performance in angiosperms—a review. Protoplasma 254:57–73. https://doi.org/10.1007/s00709-016-0950-6
Castiñeiras P, Vázquez-Ruiz RA, Fernández-González M, Rodríguez-Rajo FJ, Aira MJ (2019) Production and viability of Fraxinus pollen and its relationship with aerobiological data in the northwestern Iberian Peninsula. Aerobiologia 35:227–241. https://doi.org/10.1007/s10453-018-09553-z
Choudhary N, Siddiqui MB, Bi S, Khatoon S (2014) Effect of seasonality and time after anthesis on the viability and longevity of Cannabis sativa pollen. J Palynol 38(2):235–241. https://doi.org/10.1080/01916122.2014.892906
Chouvy PA (2008) Production de cannabis et de haschich au Maroc: contexte et enjeux. L’espace Polit Rev Ligne Géogr Polit Géopolit 4:59. https://doi.org/10.4000/espacepolitique.59
Chouvy PA, Afsahi K (2014) Hashish revival in Morocco. Int J Drug Policy 25(3):416–423. https://doi.org/10.1016/j.drugpo.2014.01.001
Chouvy PA, Macfarlane J (2018) Agricultural innovations in Morocco’s cannabis industry. Int J Drug Policy 58:85–91. https://doi.org/10.1016/j.drugpo.2018.04.013
Clarke R, Merlin M (2016) Cannabis: evolution and ethnobotany. University of California Press, Oakland. https://doi.org/10.1525/j.ctt3fh2f8
Dafni A (1992) Pollination ecology: a practical approach. Oxford University Press, Oxford. https://doi.org/10.2307/2807163
Dingha BN, Jackai LE (2025) The potential impact of flower characteristics and pollen viability of four industrial hemp (Cannabis sativa L.) grain varieties on cross-pollination. Agronomy 15:515. https://doi.org/10.3390/agronomy15030515
El Bakali I, Hassoun M, Boutahar A, El Bakali S, Sakar EH, Kadiri M, Merzouki A (2024) A comparative evaluation of biomass and resin by-products attributes of six hemp (Cannabis sativa L.) cultivars grown in Rif Mountains (northern Morocco). Vegetos 45:1–13. https://doi.org/10.1007/s42535-024-01077-x
Faegri K, Iversen J (1989) Textbook of Pollen Analysis. Wiley, Chichester. https://doi.org/10.1002/jqs.3390050310
Flajšman M, Slapnik M, Murovec J (2021) Production of feminized seeds of high CBD Cannabis sativa L. by manipulation of sex expression and its application to breeding. Front Plant Sci 12:718092. https://doi.org/10.3389/fpls.2021.718092
Freeman DC, Harper KT, Charnov EL (1980) Sex change in plants: old and new observations and new hypotheses. Oecologia 47:222–232. https://doi.org/10.1007/BF00346825
Gaudet D, Yadav NS, Sorokin A, Bilichak A, Kovalchuk I (2020) Development and optimization of a germination assay and long-term storage for Cannabis sativa pollen. Plants 9(5):665. https://doi.org/10.3390/plants9050665
Gómez-Mena C, Honys D, Datla R, Testillano PS (2022) Advances in pollen research: biology, biotechnology, and plant breeding applications. Front Plant Sci 13:876502. https://doi.org/10.3389/fpls.2022.876502
Iovane M, Izzo LG, Cirillo A, Romano LE, Di Vaio C, Aronne G (2022) Flowering and pollen resilience to high temperature of apricot cultivars. Sci Hortic 304:111261. https://doi.org/10.1016/j.scienta.2022.111261
Jayaprakash P, Sarla N (2001) Development of an improved medium for germination of Cajanus cajan (L.) Millsp. pollen in vitro. J Exp Bot 52(357):851–855. https://doi.org/10.1093/jexbot/52.357.851
Käfer J, Méndez M, Mousset S (2022) Labile sex expression in angiosperm species with sex chromosomes. Philos Trans R Soc Lond B Biol Sci 377(1850):20210216. https://doi.org/10.1098/rstb.2021.0216
Kim J, Kim DG, Kim WJ, Lee YJ, Lee SH, Ryu J, Kim JH, Kim SH (2024) Characterization of male flower induction by silver thiosulfate foliar spray in female Cannabis at the middle reproductive stage for breeding. Plants 13:2429. https://doi.org/10.3390/plants13172429
Kouboris GC, Metzidakis IT, Vasilakakis MD (2012) Intraspecific variation in pollen viability, germination and ultrastructure of Olea europaea L. Afr J Biotechnol 11:13442–13446. https://doi.org/10.5897/AJB12.626
Love J, Selker R, Marsman M, Jamil T, Dropmann D, Verhagen J, Ly A, Gronau QF, Smíra M, Epskamp S, Matzke D, Wild A, Knight P, Rouder JN, Morey RD, Wagenmakers E (2019) JASP: graphical statistical software for common statistical designs. J Stat Softw 88(2):1–17. https://doi.org/10.18637/jss.v088.i02
Merzouki A (2001) El cultivo del cáñamo (Cannabis sativa L.) en el RIF, norte de Marruecos; taxonomía, biología y etnobotánica. Universidad de Granada, Granada
Mesnoua M, Mezerdi F, Belouz K, Guerbaze K, Roumani M, Faci M, Foughalia A, Bettiche F, Nia B, Tahirine M, Ouamane AT (2024) The influence of temperature on pollen germination and pollen tube growth in eight date palm cultivars. Agric Res. https://doi.org/10.1007/s40003-024-00726-6
Metouekel A, Badrana F, Kachkoul R, Chebaibi M, Akhazzane M, El Moussaoui A, Touil N, El Amri H, El Fahime E, El Kazzouli S, El Brahmi N (2024) Genetic characterization and chemical identification of Moroccan Cannabis sativa (L.) seeds: extraction, and in vitro and in silico biological evaluation. Plants 13(14):1938. https://doi.org/10.3390/plants13141938
NASA-National Aeronautics and Space Administration (2024) Power data access viewer: prediction of worldwide energy resource. https://power.larc.nasa.gov/data-access-viewer/. Accessed 22 Sept 2024.
Nath MK (2022) Benefits of cultivating industrial hemp (Cannabis sativa ssp. sativa)—a versatile plant for a sustainable future. Chem Proc 10(1):14. https://doi.org/10.3390/IOCAG2022-12359
Ouhtit R, Ouhtit A, Redouan FZ, Lamrani Z, Merzouki A (2024) Morphometry, oil yield and fatty acid profile of Cannabis Achenes from the Chefchaouen Region. Mor J Chem 12(1):12–21. https://doi.org/10.48317/IMIST.PRSM/morjchem-v12i1.42058
Rafiq H, Hartung J, Burgel L, Röll G, Graeff-Hönninger S (2021) Potential of impedance flow cytometry to assess the viability and quantity of Cannabis sativa L. pollen. Plants 10(12):2739. https://doi.org/10.3390/plants10122739
Rana A, Choudhary N (2010) Floral biology and pollination biology of Cannabis sativa L. Int J Plant Reprod Biol 2(2):191–195
Ren R, Li Z, Jiang X, Liu Y (2020) The ROS-associated programmed cell death causes the decline of pollen viability recovered from cryopreservation in Paeonia lactiflora. Plant Cell Rep 39:941–952
Rodriguez-Riano T, Dafni A (2000) A new procedure to asses pollen viability. Sex Plant Reprod 12:241–244. https://doi.org/10.1007/s004970050008
Rojo J, Salido P, Pérez-Badia R (2015) Flower and pollen production in the ‘Cornicabra’ olive (Olea europaea L.) cultivar and the influence of environmental factors. Trees 29:1235–1245. https://doi.org/10.1007/s00468-015-1203-6
Schilling S, Melzer R, Dowling CA, Shi J, Muldoon S, McCabe PF (2023) A protocol for rapid generation cycling (speed breeding) of hemp (Cannabis sativa) for research and agriculture. Plant J 113(3):437–445. https://doi.org/10.1111/tpj.16051
Shivanna KR, Rangaswamy NS (2012) Pollen biology: a laboratory manual. Springer, Cham. https://doi.org/10.1007/978-3-642-77306-8
Small E (2015) Evolution and classification of Cannabis sativa (marijuana, hemp) in relation to human utilization. Bot Rev 81:189–294. https://doi.org/10.1007/s12229-015-9157-3
Small E (2016) Cannabis–a complete guide. CRC Press, Taylor & Francis Group, Boca. https://doi.org/10.1201/9781315367583
Small E, Pocock T, Cavers PB (2003) The biology of Canadian weeds. 119. Cannabis sativa L. Can J Plant Sci 83(1):217–237. https://doi.org/10.4141/P02-021
Sulusoglu M, Cavusoglu A (2014) In vitro pollen viability and pollen germination in cherry laurel (Prunus laurocerasus L.). Sci World J 2014(1):657123. https://doi.org/10.1155/2014/657123
Thuzar M (2010) The effects of temperature stress on the quality and yield of Soya bean [(Glycine max L.) Merrill]. J Agric Sci. https://doi.org/10.5539/jas.v2n1p172
Tuinstra MR, Wedel J (2000) Estimation of pollen viability in grain sorghum. Crop Sci 40(4):968–970. https://doi.org/10.2135/cropsci2000.404968x
UNODC (2022) World drug report 2022. UNODC, Vienna
Vanhove W, Van Damme P, Meert N (2011) Factors determining yield and quality of illicit indoor cannabis (Cannabis spp.) production. Forensic Sci Int 212(1–3):158–163. https://doi.org/10.1016/j.forsciint.2011.06.006
Warf B (2014) High points: an historical geography of cannabis. Geogr Rev 104(4):414–438. https://doi.org/10.1111/j.1931-0846.2014.12038.x
Wizenberg SB, Dang M, Campbell LG (2022) Methods for characterizing pollen fitness in Cannabis sativa L. PLoS ONE 17(7):e0270799. https://doi.org/10.1371/journal.pone.0270799
Wizenberg SB, Muir-Guarnaccia J, Campbell LG (2023) Cosexuality reduces pollen production and fitness in Cannabis sativa L. Plants 12(21):3731. https://doi.org/10.3390/plants12213731
Zhatov AI (1983) Viability of pollen grains of polyploid hemp. Tsitol Genet 17(1):47–51
Author Information
Biology, Ecology, and Health Laboratory, Department of Biology, Faculty of Sciences of Tetouan, Abdelmalek Essaâdi University, Tétouan, Morocco