Polyphenols from an endemic Algerian medicinal plant: Henophyton deserti, optimization and evaluation of biological activities
*Article not assigned to an issue yet
Research Articles | Published: 18 December, 2025
First Page: 0
Last Page: 0
Views: 614
Keywords: n Henophyton desertin , Microwave-assisted extraction, Ultrasound-assisted extraction, Response surface methodology, Phenolic compounds
Abstract
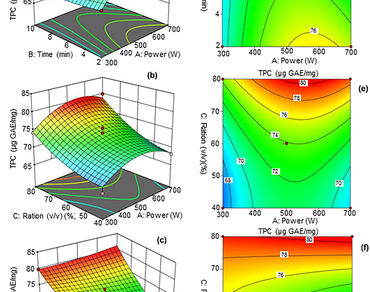
Polyphenols from Henophyton deserti (H.deserti) were extracted using microwave-assisted extraction (MAE) and ultrasound-assisted extraction (UAE). Response surface methodology (RSM) employing a Box-Behnken design was utilized. This approach optimized the extraction parameters for total phenolic concentration (TPC). The independent variables for (MAE) included power in (W), extraction duration (min), and methanol concentration (%), while amplitude (%), irradiation time (min), and methanol concentration were used for UAE. Optimal conditions for MAE were 561.18 W, 2.77 min, and 79.32% methanol concentration, while UAE required 30.13% amplitude, 16.82 min, and 79.57% methanol concentration. Predicted TPC recoveries were 80.05 µg GAE/mg (MAE) and 77.71 µg GAE/mg (UAE), which were experimentally validated with values of 79.89 ± 0.80 and 75.86 ± 0.65 µg GAE/mg, respectively. Antioxidant activity, assessed by assay (diphenyl-1-picrylhydrazyl) DPPH, hydroxyl radical scavenging, ferric reducing antioxidant power FRAP, and phenanthroline assays, confirmed MAE’s suitability for obtaining antioxidant-rich extracts. At the same time, the UAE demonstrated higher efficacy for antimicrobial activity.
References
Alara OR, Abdurahman NH, Ali HA, Zain NM (2021) Microwave-assisted extraction of phenolic compounds from carica Papaya leaves: an optimization study and LC-QTOF-MS analysis. Future Foods 3:100035. https://doi.org/10.1016/j.fufo.2021.100035
Aliaño-González MJ, Barea-Sepúlveda M, Espada-Bellido E, Ferreiro-González M, López-Castillo JG, Palma M, Barbero GF, Carrera C (2022) Ultrasound-Assisted extraction of total phenolic compounds and antioxidant activity in mushrooms. Agronomy 12:1812. https://doi.org/10.3390/agronomy12081812
Bayarsaikhan G, Dondurmacioglu F, Avan AN, Cekic SD, Apak R (2018) Novel colorimetric assay of 2,3-Dihydroxybenzoate among other isomers as a selective indicator of hydroxyl radical damage and related antioxidant activity. Anal Lett 51:236–253. https://doi.org/10.1080/00032719.2017.1328689
Bensaci C, Belguidoum M, Khattabi L, Abid A, Touahria T, Zahnit W, Harchaoui L, Rahmani Z, Boussebaa W, Laichi Y, Belfar A, Farah MA, Al-Anazi KM, Ali A (2024) Drimia maritima flowers as a source of biologically potent components: optimization of bioactive compound extractions, isolation, UPLC–ESI–MS/MS, and Pharmacological properties. Open Chem 22:20240087. https://doi.org/10.1515/chem-2024-0087
Biswas R, Sarkar A, Alam M, Roy M, Mahdi Hasan MM (2023) Microwave and ultrasound-assisted extraction of bioactive compounds from papaya: A sustainable green process. Ultrason Sonochem 101:106677. https://doi.org/10.1016/j.ultsonch.2023.106677
Boukezzoula A, Boudemagh D, Palko N, Grishina M, Bensouici C, Bounekhel M, AlShamaileh E, Dahamna S (2024) Quantification, phytochemical evaluation, and identification of pomegranate yellow Peel constituents using LC-MS/MS, the effect of the solvent extract on the antioxidant, in vitro anti-inflammatory, and antibacterial activities. Ind Crops Prod 219:118780. https://doi.org/10.1016/j.indcrop.2024.118780
Bramki A, Benouchenne D, Salvatore MM, Benslama O, Andolfi A, Rahim N, Moussaoui M, Ramoul S, Nessah S, Barboucha G, Bensouici C, Cimmino A, Zorrilla JG, Masi M (2024) In vitro and in Silico biological activities investigation of Ethyl acetate extract of rubus ulmifolius Schott leaves collected in Algeria. Plants 13:3425. https://doi.org/10.3390/plants13233425
Chaachouay N, Zidane L (2024) Plant-Derived natural products: A source for drug discovery and development. Drugs Drug Candidates 3:184–207. https://doi.org/10.3390/ddc3010011
Dahmoune F, Boulekbache L, Moussi K, Aoun O, Spigno G, Madani K (2013) Valorization of Citrus Limon residues for the recovery of antioxidants: evaluation and optimization of microwave and ultrasound application to solvent extraction. Ind Crops Prod 50:77–87. https://doi.org/10.1016/j.indcrop.2013.07.013
Dahmoune F, Nayak B, Moussi K, Remini H, Madani K (2015) Optimization of microwave-assisted extraction of polyphenols from Myrtus communis L leaves. Food Chem 166:585–595. https://doi.org/10.1016/j.foodchem.2014.06.066
Davis CC, Choisy P (2024) Medicinal plants Meet modern biodiversity science. Curr Biol 34:R158–R173. https://doi.org/10.1016/j.cub.2023.12.038
Derbel S, Bouaziz M, Dhouib A, Sayadi S, Chaieb M (2010) Chemical composition and biological potential of seed oil and leaf extracts of Henophyton deserti Coss. Comptes Rendus Chim 13:473–480. https://doi.org/10.1016/j.crci.2009.10.004. Durieu
Derouiche S, Iman S, Maroua Z, Mehellou Z (2020) Anti-diabetic and Anti-oxidative activities of Algerian Oudneya Africana extract in Alloxan. Induc Diabet Rats 2:137–145. https://doi.org/10.0000/ajer.2020.137.145
Dewi SR, Stevens LA, Pearson AE, Ferrari R, Irvine DJ, Binner ER (2022) Investigating the role of solvent type and microwave selective heating on the extraction of phenolic compounds from Cacao (Theobroma Cacao L.) pod husk. Food Bioprod Process 134:210–222. https://doi.org/10.1016/j.fbp.2022.05.011
Djemaa-Landri K, Hamri-Zeghichi S, Belkhiri-Beder W, Krisa S, Cluzet S, Richard T, Valls J, Kadri N, Madani K (2021) Phenolic content, antioxidant and anti-inflammatory activities of some Algerian Olive stone extracts obtained by conventional solvent and microwave-assisted extractions under optimized conditions. J Food Meas Charact 15:4166–4180. https://doi.org/10.1007/s11694-021-00992-w
Elez Garofulić I, Dragović-Uzelac V, Režek Jambrak A, Jukić M (2013) The effect microwave assisted extraction on the isolation of anthocyanins and phenolic acids from sour Cherry Marasca (Prunus cerasus var. Marasca). J Food Eng 117:437–442. https://doi.org/10.1016/j.jfoodeng.2012.12.043
Elshafie HS, Camele I, Mohamed AA (2023) A comprehensive review on the Biological, agricultural and pharmaceutical properties of secondary metabolites Based-Plant origin. Int J Mol Sci 24:3266. https://doi.org/10.3390/ijms24043266
Fang C, Luo J, Wang S (2019) The diversity of nutritional metabolites: Origin, Dissection, and application in crop breeding. Front Plant Sci 10. https://doi.org/10.3389/fpls.2019.01028
Garcia-Vaquero M, Ravindran R, Walsh O, O’Doherty J, Jaiswal AK, Tiwari BK, Rajauria G (2021) Evaluation of Ultrasound, Microwave, Ultrasound–Microwave, hydrothermal and high pressure assisted extraction technologies for the recovery of phytochemicals and antioxidants from brown macroalgae. Mar Drugs 19:309. https://doi.org/10.3390/md19060309
Gavarić A, Vladić J, Vujetić J, Radnović D, Volarić A, Živković J, Šavikin K, Vidović S (2022) The application of ultrasonic waves and microwaves to improve antihyperglycaemic and antimicrobial activities of marrubium vulgare extracts. Antibiotics 11:1475. https://doi.org/10.3390/antibiotics11111475
Hajlaoui H, Arraouadi S, Mighri H, Chaaibia M, Gharsallah N, Ros G, Nieto G, Kadri A (2019) Phytochemical constituents and antioxidant activity of Oudneya Africana L. Leaves extracts: evaluation effects on fatty acids and proteins oxidation of beef burger during refrigerated storage. Antioxidants 8:442. https://doi.org/10.3390/antiox8100442
Hamdani D, Benamar H, Bennaceur M, Chouh A, Bensouici C (2025) In vitro antioxidant and enzyme inhibitory Properties, phenolic contents and UHPLC-ESI/MSMS analysis of aerial parts of anacyclus Valentinus L. from the high plateaus of Algeria (Brezina Province). Waste Biomass Valorization 16:3557–3573. https://doi.org/10.1007/s12649-024-02881-x
Hammami R, Hamida J, Vergoten G, Lacroix JM, Slomianny MC, Neffati M, Fliss I (2009) A new antimicrobial peptide isolated from Oudneya Africana seeds. Microbiol Immunol 53:658–666. https://doi.org/10.1111/j.1348-0421.2009.00183.x
Harel O (2009) The Estimation of R2 and adjusted R2 in incomplete data sets using multiple imputation. J Appl Stat 36:1109–1118. https://doi.org/10.1080/02664760802553000
Ince A, Sahin S, Sumnu G (2013) Extraction of phenolic compounds from Melissa using microwave and ultrasound. Turk J Agric for 37:69–75. https://doi.org/10.3906/tar-1201-1
Ioannou I, Chaaban H, Slimane M, Ghoul M, Ekinci D (2015) Origin of the variability of the antioxidant activity determination of food material. Biotechnol IntechOpen. https://doi.org/10.5772/60453
Ismail BB, Guo M, Pu Y, Wang W, Ye X, Liu D (2019) Valorisation of baobab (Adansonia digitata) seeds by ultrasound assisted extraction of polyphenolics. Optimisation and comparison with conventional methods. Ultrason Sonochem 52:257–267. https://doi.org/10.1016/j.ultsonch.2018.11.023
Jerman T, Trebše P, Mozetič Vodopivec B (2010) Ultrasound-assisted solid liquid extraction (USLE) of Olive fruit (Olea europaea) phenolic compounds. Food Chem 123:175–182. https://doi.org/10.1016/j.foodchem.2010.04.006
Jomova K, Alomar SY, Valko R, Liska J, Nepovimova E, Kuca K, Valko M (2025) Flavonoids and their role in oxidative stress, inflammation, and human diseases. Chem Biol Interact 413:111489. https://doi.org/10.1016/j.cbi.2025.111489
Khan MK, Ahmad K, Hassan S, Imran M, Ahmad N, Xu C (2018) Effect of novel technologies on polyphenols during food processing. Innov Food Sci Emerg Technol 45:361–381. https://doi.org/10.1016/j.ifset.2017.12.006
Kiptiyah S, Santoso U, Supriyadi S, Harmayani E (2023) Effect of heat treatment on antioxidant and antibacterial activity of Kaempferia Galanga L. extract. Food Res 7:205–213. https://doi.org/10.26656/fr.2017.7(6).1002
Lovrić V, Putnik P, Kovačević DB, Jukić M, Dragović-Uzelac V (2017) Effect of Microwave-Assisted extraction on the phenolic compounds and antioxidant capacity of Blackthorn flowers. Food Technol Biotechnol 55:243. https://doi.org/10.17113/ftb.55.02.17.4687
Mahboub N, Slimani N (2017) Study of the effect of different drying modes on the antioxidant and antibacterial activities of Oudneya Africana. Pharmacophor 6–9. https://pharmacophorejournal.com/OUuXpaX
Mandal V, Mohan Y, Hemalatha S (2006) Microwave assisted extraction - An innovative and promising extraction tool for medicinal plant research. Pharmacogn Rev 1. https://www.researchgate.net/publication/237218276
Markus K, Kirschbaum T, Metzsch-Zilligen E, Pfaendner R (2025) Processing stability and radical scavenging efficiency of novel biobased stabilizers: insights from long-term extrusion and DPPH assays. Polym Degrad Stab 233:111162. https://doi.org/10.1016/j.polymdegradstab.2024.111162
Martínez-Olivo AO, Carlos-Murillo MU, Sáyago-Ayerdi SG, Sánchez-Burgos JA, Zamora-Gasga VM (2023) Optimization of ultrasonic extraction for enhanced polyphenol profile and antioxidant capacity in Mango seeds: A comparative study with thermal extraction. Food Chem Adv 3:100480. https://doi.org/10.1016/j.focha.2023.100480
Michel T (2011) Nouvelles méthodologies d’extraction, de fractionnement et d’identification: application aux molécules bioactives de l’argousier (Hippophae rhamnoides) (phdthesis). Université d’Orléans. https://theses.hal.science/tel-00677211v1
Mohammed EA, Abdalla IG, Alfawaz MA, Mohammed MA, Al Maiman SA, Osman MA, Yagoub AEA, Hassan AB (2022) Effects of extraction solvents on the total phenolic Content, total flavonoid Content, and antioxidant activity in the aerial part of root vegetables. Agriculture 12:1820. https://doi.org/10.3390/agriculture12111820
Muñiz-Márquez DB, Martínez-Ávila GC, Wong-Paz JE, Belmares-Cerda R, Rodríguez-Herrera R, Aguilar CN (2013) Ultrasound-assisted extraction of phenolic compounds from Laurus nobilis L.and their antioxidant activity. Ultrason Sonochem 20:1149–1154. https://doi.org/10.1016/j.ultsonch.2013.02.008
Nabti Z, Belhattab R (2016) In vitro antioxidant activity of Oudneya Africana R. Br. aerial parts. Issues Biol Sci Pharm Res 4:58–64. https://doi.org/10.15739/ibspr.16.008i
Nana O, Momeni J, Boyom FF, Njintang NY, Ngassoum MB (2021) Microwave-assisted extraction as an advanced technique for optimisation of limonoid yields and antioxidant potential from Trichilia Roka (Meliaceae). Curr Res Green Sustain Chem 4:100147. https://doi.org/10.1016/j.crgsc.2021.100147
Nguyen N, Duong H, Bao Long H, Nhi T, Dao TP (2020) Effects of microwave extraction conditions on polyphenol content and antioxidant activity of pomelo extract (Citrus maxima (Burm.)Merr). IOP Conf Ser Mater Sci Eng 991:012035. https://doi.org/10.1088/1757-899X/991/1/012035
Nguyen NVT, Nguyen CV, Duong NT, Dai XTT, Nguyen KT, Le CTT (2024) Optimization of ultrasound-assisted extraction using response surface methodology and quantification of polyphenol compounds in avicennia officinalis L. from Vietnam. Pharmacia 71:1–9. https://doi.org/10.3897/pharmacia.71.e115528
Rabia SH, Debib A, Eddaikra A, Aberkane-Mchebbek L, Nouri R, Benmoussa F, Mokhtari A, Medjber S, Mansouri B, Messaoudi M (2024) Impact of gamma irradiation on phytochemical composition, and biological activities of lepidium sativum seeds extract. Radiochim Acta 112:351–362. https://doi.org/10.1515/ract-2023-0260
Rempe CS, Burris KP, Lenaghan SC, Stewart CN (2017) The potential of systems biology to discover antibacterial mechanisms of plant phenolics. Front Microbiol 8. https://doi.org/10.3389/fmicb.2017.00422
Saifullah M, McCullum R, Vuong Q (2020) Maximising extraction yields of Gallic acid and Hesperetin from lemon Myrtle (Backhousia citriodora) leaf using microwave assisted extraction. Results Chem 2:100080. https://doi.org/10.1016/j.rechem.2020.100080
Salah N, Ammar Z, Bensouici C, Amor M, Haouat A, Louafi F, Moussaoui Y, Salem R, Khan M, Ghernaout D, Elboughdiri N (2023) In vitro antioxidant and antibacterial activities of ethanolic extracts from the leaves and stems of Oudneya Africana R. growing in the El Oued (Algeria). Biomass Convers Biorefinery 14:29911–29922. https://doi.org/10.1007/s13399-023-04856-9
Sirichan T, Kijpatanasilp I, Asadatorn N, Assatarakul K (2022) Optimization of ultrasound extraction of functional compound from Makiang seed by response surface methodology and antimicrobial activity of optimized extract with its application in orange juice. Ultrason Sonochem 83:105916. https://doi.org/10.1016/j.ultsonch.2022.105916
Smith-Hall C, Larsen HO, Pouliot M (2012) People, plants and health: a conceptual framework for assessing changes in medicinal plant consumption. J Ethnobiol Ethnomed 8:1–11. https://doi.org/10.1186/1746-4269-8-43
Stocker P, Yousfi M, Salmi C, Perrier J, Brunel JM, Moulin A (2005) Maackiain 3-O-(6′-O-malonyl-β-D-glucopyranoside) from Oudneya africana, a powerful inhibitor of Porcine kidney acylase I. Biochimie 87:507–512. https://doi.org/10.1016/j.biochi.2005.02.010
Sun Y, Lu J, Li J, Li P, Zhao M, Xia G (2023) Optimization of ultrasonic-assisted extraction of polyphenol from Areca nut (Areca catechu L.) seeds using response surface methodology and its effects on osteogenic activity. Ultrason Sonochem 98:106511. https://doi.org/10.1016/j.ultsonch.2023.106511
Talbi S, Romero-Puertas MC, Hernández A, Terrón L, Ferchichi A, Sandalio LM (2015) Drought tolerance in a Saharian plant Oudneya africana: role of antioxidant defences. Environ Exp Bot 111:114–126. https://doi.org/10.1016/j.envexpbot.2014.11.004
Telli A, Esnault MA, Ould E, Hadj Khelil A (2016) An ethnopharmacological survey of plants used in traditional diabetes treatment in south-eastern Algeria (Ouargla province). J Arid Environ 127:82–92. https://doi.org/10.1016/j.jaridenv.2015.11.005
Wu Y, Bai J, Zhong K, Huang Y, Qi H, Jiang Y, Gao H (2016) Antibacterial activity and Membrane-Disruptive mechanism of 3-p-trans-Coumaroyl-2-hydroxyquinic Acid, a novel phenolic compound from pine needles of cedrus deodara, against Staphylococcus aureus. Mol Basel Switz 21:1084. https://doi.org/10.3390/molecules21081084
Wu Y, Liang S, Zhang M, Wang Z, Ziyuan W, Ren X (2020) The effect of chlorogenic acid on Bacillus subtilis based on metabolomics. Mol Basel Switz 25:4038. https://doi.org/10.3390/molecules25184038
Xiang Y, Liu Z, Liu Y, Dong B, Yang C, Li H (2024) Ultrasound-assisted extraction, optimization, and purification of total flavonoids from Daphnegenkwa and analysis of their antioxidant, anti-inflammatory, and analgesic activities. Ultrason Sonochem 111:107079. https://doi.org/10.1016/j.ultsonch.2024.107079
Xiaokang W, Lyng JG, Brunton NP, Cody L, Jacquier JC, Harrison SM, Papoutsis K (2020) Monitoring the effect of different microwave extraction parameters on the recovery of polyphenols from Shiitake mushrooms: comparison with hot-water and organic-solvent extractions. Biotechnol Rep 27:e00504. https://doi.org/10.1016/j.btre.2020.e00504
Yang X, Wang L, Dong C, Lui EMK, Ren G (2014) Optimization of Ultrasonic-Assisted extraction process of polysaccharides from American ginseng and evaluation of its immunostimulating activity. J Integr Agric 13:2807–2815. https://doi.org/10.1016/S2095-3119(14)60785-1
Zahnit W, Smara O, Bechki L, Bensouici C, Messaoudi M, Benchikha N, Larkem I, Awuchi CG, Sawicka B, Simal-Gandara J (2022) Phytochemical Profiling, mineral Elements, and biological activities of Artemisia Campestris L. Grown in Algeria. Horticulturae 8:914. https://doi.org/10.3390/horticulturae8100914
Zou P, Costas C, Jyakhwo S, Cameselle C, Otero P (2025) Guidelines for the extraction of plant polyphenols using Microwave-Assisted techniques. Food Chem Int 1:351–361. https://doi.org/10.1002/fci2.70022
Zahorec J, Šoronja-Simović D, Kocić-Tanackov S, Bulut S, Martić N, Bijelić K, Božović D, Pavlić B (2023) Carob pulp flour extract obtained by a Microwave-Assisted extraction technique: A prospective antioxidant and antimicrobial agent. Separations 10:465. https://doi.org/10.3390/separations10090465
Zhang S, Xie H, Huang J, Chen Q, Li X, Chen X, Liang J, Wang L (2024) Ultrasound-assisted extraction of polyphenols from pine needles (Pinus elliottii): comprehensive insights from RSM optimization, antioxidant activity, UHPLC-Q-Exactive orbitrap MS/MS analysis and kinetic model. Ultrason Sonochem 102:106742. https://doi.org/10.1016/j.ultsonch.2023.106742
Author Information
Bioactive Molecules and Chiral Separation Laboratory, Faculty of Exact Sciences, University Tahri Mohamed of Bechar, Bechar, Algeria