Potential use of phosphate solubilizing actinobacteria as plant enhancers: involvement in plant nutrition and stress tolerance
*Article not assigned to an issue yet
Bousselham Meriam, Aallam Yassine, Dhiba Driss, Abbas Younes, Saidi Nezha, Hamdali Hanane
Research Articles | Published: 31 July, 2025
First Page: 0
Last Page: 0
Views: 110
Keywords: Actinomycetes, Oat, Plant growth-promoting traits, Stress tolerance, Sustainable agriculture
Abstract
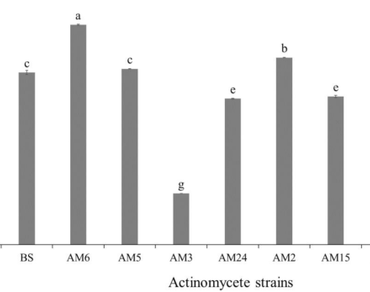
Eight oat rhizospheric actinomycetes (AM1, AM2, AM3, AM5, AM6, AM13, AM15, and AM24) have been characterized in our previous study for their phosphate solubilization abilities, siderophore production, and genetic diversity. In the present study, the eight selected strains were characterized for their plant growth promotion traits through several mechanisms. Results showed that the most active indole acetic acid (IAA) producers are Streptomyces anulatus (AM6), Streptomyces griseoflavus (AM2) and Streptomyces sampsonii (AM5) (37.64 µg.mL− 1, 32.03 µg.mL− 1 and 30.10 µg.mL− 1, respectively). All the strains were able to release soluble potassium from orthoclase and mica, and they were able to tolerate high concentrations of polyethylene glycol (PEG) 6000 (25%), which refers to drought stress tolerance. Only AM6, AM24, AM2, AM5, AM15, and AM13 produced hydrogen cyanide (HCN). Five strains were able to produce ammonia, whereas all were able to fix nitrogen. Moreover, the eight strains conferred biotic stress tolerance through resistance to several pathogens (Gram-positive and Gram-negative bacteria, yeast, and fungi). Furthermore, they were able to resist several synthetic antibiotics that limit pathogen growth. The most active strains among the tested Actinomycetes were AM2, AM5, and AM6. Our strains contribute to plant growth by facilitating biotic and abiotic stress tolerance and supporting plant nutrition. This could be considered in the framework of a biological approach to sustainable agriculture.
References
Aallam Y, Dhiba D, El Rasafi T et al (2022) Growth promotion and protection against root rot of sugar beet (Beta vulgaris L.) by two rock phosphate and potassium solubilizing streptomyces spp. Under greenhouse conditions. Plant Soil 472:407–420. https://doi.org/10.1007/s11104-021-05252-w
Aallam Y, Dhiba D, Lemriss S et al (2021a) Isolation and characterization of phosphate solubilizing streptomyces sp. Endemic from sugar beet fields of the beni-mellal region in Morocco. Microorganisms 9. https://doi.org/10.3390/microorganisms9050914
Aallam Y, Dhiba D, Rasafi T, El et al (2023) Assessment of two endemic rock phosphate solubilizing streptomyces spp. On sugar beet (Beta vulgaris L.) growth under field conditions. Sci Hortic (Amsterdam) 316:112033. https://doi.org/10.1016/j.scienta.2023.112033
Aallam Y, El Maliki B, Dhiba D et al (2021b) Multiple potential plant growth promotion activities of endemic streptomyces spp. From Moroccan sugar beet fields with their inhibitory activities against fusarium spp. Microorganisms 9:1–15. https://doi.org/10.3390/microorganisms9071429
Abdelgawad H, Abuelsoud W, Madany MMY et al (2020) Actinomycetes enrich soil rhizosphere and improve seed quality as well as productivity of legumes by boosting nitrogen availability and metabolism. Biomolecules 10:1–19. https://doi.org/10.3390/biom10121675
Adegboye MF, Babalola OO (2013) Actinomycetes: a yet inexhaustive source of bioactive secondary metabolites. Microb Pathog Strateg Combat Them Sci Technol Educ 19:786–795. https://doi.org/10.1186/s43141-021-00156-9
Ahmed B, Shahid M, Syed A et al (2021) Drought tolerant Enterobacter sp./leclercia adecarboxylata secretes indole-3-acetic acid and other biomolecules and enhances the biological attributes of vigna radiata (l.) r. wilczek in water deficit conditions. Biology (Basel) 10. https://doi.org/10.3390/biology10111149
Anwar S, Ali B, Sajid I (2016) Screening of rhizospheric actinomycetes for various in-vitro and in-vivo plant growth promoting (PGP) traits and for agroactive compounds. Front Microbiol 7:1–11. https://doi.org/10.3389/fmicb.2016.01334
Baba IA, Wani ZA, Ali S et al (2025) Actinomycetes - The repertoire of diverse bioactive chemical molecules: from structures to antibiotics. Curr Top Med Chem 25:986–998
Babalola OO, Glick BR (2012) The use of microbial inoculants in African agriculture: current practice and future prospects. J Food Agric Environ 10:540–549
Bakker AW, Schippers B (1987) Microbial cyanide production in the rhizosphere in relation to potato yield reduction and Pseudomonas SPP-mediated plant growth-stimulation. Soil Biol Biochem 19:451–457. https://doi.org/10.1016/0038-0717(87)90037-X
Baldani JI, Reis VM, Videira SS et al (2014) The Art of isolating nitrogen-fixing bacteria from non-leguminous plants using N-free semi-solid media: a practical guide for microbiologists. Plant Soil 384:413–431. https://doi.org/10.1007/s11104-014-2186-6
Boubekri K, Soumare A, Mardad I et al (2021) The screening of potassium-and phosphate-solubilizing actinobacteria and the assessment of their ability to promote wheat growth parameters. Microorganisms 9:1–16. https://doi.org/10.3390/microorganisms9030470
Bousselham M, Lemriss S, Dhiba D et al (2022) Streptomycetaceae and promicromonosporaceae: two actinomycetes families from Moroccan oat soils enhancing solubilization of natural phosphate. https://doi.org/10.3390/microorganisms10061116. Microorganisms 10:
Castillo-Alfonso F, Quintana-Menéndez A, Vigueras-Ramírez G et al (2022) Analysis of the propionate metabolism in Bacillus subtilis during 3-Indolacetic production. https://doi.org/10.3390/microorganisms10122352. Microorganisms 10:
Chandra D, Srivastava R, Gupta VSR et al (2019) Evaluation of ACC deaminase producing rhizobacteria to alleviate water stress impacts authors. Can J Microbiol 65:387–403
Chaurasia A, Meena BR, Tripathi AN et al (2018) Actinomycetes: an unexplored microorganisms for plant growth promotion and biocontrol in vegetable crops. World J Microbiol Biotechnol 34:1–16. https://doi.org/10.1007/s11274-018-2517-5
Chukwuneme CF, Babalola OO, Kutu FR, Ojuederie OB (2020) Characterization of actinomycetes isolates for plant growth promoting traits and their effects on drought tolerance in maize. J Plant Interact 15:93–105. https://doi.org/10.1080/17429145.2020.1752833
De Simeis D, Serra S (2021) Actinomycetes: A never-ending source of bioactive compounds—an overview on antibiotics production. Antibiotics 10. https://doi.org/10.3390/antibiotics10050483
Dicko AH, Babana AH, Kassogué A et al (2018) A Malian native plant growth promoting actinomycetes based biofertilizer improves maize growth and yield. Symbiosis 75:267–275. https://doi.org/10.1007/s13199-018-0555-2
Domagal-Goldman SD, Paul KW, Sparks DL, Kubicki JD (2009) Quantum chemical study of the Fe(III)-desferrioxamine B siderophore complex-Electronic structure, vibrational frequencies, and equilibrium Fe-isotope fractionation. Geochim Cosmochim Acta 73:1–12. https://doi.org/10.1016/j.gca.2008.09.031
Ezeobiora CE, Igbokwe NH, Amin DH et al (2022) Uncovering the biodiversity and biosynthetic potentials of rare actinomycetes. Futur J Pharm Sci 8. https://doi.org/10.1186/s43094-022-00410-y
Farda B, Djebaili R, Vaccarelli I et al (2022) Actinomycetes from caves: an overview of their diversity, biotechnological properties, and insights for their use in soil environments. https://doi.org/10.3390/microorganisms10020453. Microorganisms 10:
Gang S, Sharma S, Saraf M et al (2020) Analysis of Indole-3-acetic acid (IAA) production in Klebsiella by LC-MS/MS and the Salkowski method. J Food Legum 33:181–190. https://doi.org/10.21769/BioProtoc.3230
Gouda S, Kerry RG, Das G et al (2018) Revitalization of plant growth promoting rhizobacteria for sustainable development in agriculture. Microbiol Res 206:131–140. https://doi.org/10.1016/j.micres.2017.08.016
Grover M, Bodhankar S, Sharma A et al (2021) PGPR mediated alterations in root traits: way toward sustainable crop production. Front Sustain Food Syst 4:1–28. https://doi.org/10.3389/fsufs.2020.618230
Gupta S, Pandey S (2019) ACC deaminase producing bacteria with multifarious plant growth promoting traits alleviates salinity stress in French bean (Phaseolus vulgaris) plants. Front Microbiol 10:1–17. https://doi.org/10.3389/fmicb.2019.01506
Hamdali H, Bouizgarne B, Hafidi M et al (2008) Screening for rock phosphate solubilizing actinomycetes from Moroccan phosphate mines. Appl Soil Ecol 38:12–19. https://doi.org/10.1016/j.apsoil.2007.08.007
Hamdali H, Lebrihi A, Monje MC et al (2021) A molecule of the viridomycin family originating from a streptomyces griseus-related strain has the ability to solubilize rock phosphate and to inhibit microbial growth. Antibiotics 10:1–9. https://doi.org/10.3390/antibiotics10010072
He DC, He MH, Amalin DM et al (2021) Biological control of plant diseases: an evolutionary and eco-economic consideration. Pathogens 10. https://doi.org/10.3390/pathogens10101311
Husen E, Wahyudi AT, Suwanto A, Giyanto (2011) Growth enhancement and disease reduction of soybean by department of biology, faculty of mathematics and natural sciences department of plant protections, faculty of agriculture, Bogor agricultural university (IPB), jl. Agatis, Kampus IPB Dramaga B. Am J Appl Sci 8:1073–1080
Jacoby R, Peukert M, Succurro A et al (2017) The role of soil microorganisms in plant mineral nutrition—current knowledge and future directions. Front Plant Sci 8:1–19. https://doi.org/10.3389/fpls.2017.01617
Kavamura VN, Santos SN, da Silva JL et al (2013) Screening of Brazilian cacti rhizobacteria for plant growth promotion under drought. Microbiol Res 168:183–191. https://doi.org/10.1016/j.micres.2012.12.002
Khamna S, Yokota A, Peberdy JF, Lumyong S (2010) Indole-3-acetic acid production by streptomyces sp. isolated from some Thai medicinal plant rhizosphere soils. EurAsian J Biosci 4:23–32. https://doi.org/10.5053/ejobios.2010.4.0.4
Khan ZA, Siddiqui MF, Park S (2019) Current and emerging methods of antibiotic susceptibility testing. Diagnostics 9:1–17. https://doi.org/10.3390/diagnostics9020049
Kruasuwan W, Thamchaipenet A (2016) Diversity of culturable plant growth-Promoting bacterial endophytes associated with sugarcane roots and their effect of growth by Co-Inoculation of diazotrophs and actinomycetes. J Plant Growth Regul 35:1074–1087. https://doi.org/10.1007/s00344-016-9604-3
Kulkarni GB, Sanjeevkumar S, Kirankumar B et al (2013) Indole-3-acetic acid biosynthesis in fusarium delphinoides strain GPK, a causal agent of wilt in Chickpea. Appl Biochem Biotechnol 169:1292–1305. https://doi.org/10.1007/s12010-012-0037-6
Kumar P, Thakur S, Dhingra GK et al (2018) Inoculation of siderophore producing rhizobacteria and their consortium for growth enhancement of wheat plant. Biocatal Agric Biotechnol 15:264–269. https://doi.org/10.1016/j.bcab.2018.06.019
Lareen A, Burton F, Schäfer P (2016) Plant root-microbe communication in shaping root microbiomes. Plant Mol Biol 90:575–587. https://doi.org/10.1007/s11103-015-0417-8
Lemanceau P, Corberand T, Gardan L et al (1995) Effect of two plant species, flax (Linum usitatissinum L.) and tomato (Lycopersicon esculentum Mill.), on the diversity of soilborne populations of fluorescent pseudomonads. Appl Environ Microbiol 61:1004–1012. https://doi.org/10.1128/aem.61.3.1004-1012.1995
Marques APGC, Pires C, Moreira H et al (2010) Assessment of the plant growth promotion abilities of six bacterial isolates using Zea Mays as indicator plant. Soil Biol Biochem 42:1229–1235. https://doi.org/10.1016/j.soilbio.2010.04.014
Ndeddy Aka RJ, Babalola OO (2016) Effect of bacterial inoculation of strains of Pseudomonas aeruginosa, alcaligenes feacalis and Bacillus subtilis on germination, growth and heavy metal (cd, cr, and ni) uptake of brassica juncea. Int J Phytorem 18:200–209. https://doi.org/10.1080/15226514.2015.1073671
Nithyapriya S, Lalitha S, Sayyed RZ et al (2021) Production, purification, and characterization of bacillibactin siderophore of Bacillus subtilis and its application for improvement in plant growth and oil content in Sesame. Sustain 13. https://doi.org/10.3390/su13105394
Olomitutu OE, Abe A, Oyatomi OA et al (2022) Assessing intraspecific variability and diversity in African. Yam Bean Landraces Using Agronomic Traits
Oracz K, Bouteau HEM, Farrant JM et al (2007) ROS production and protein oxidation as a novel mechanism for seed dormancy alleviation. Plant J 50:452–465. https://doi.org/10.1111/j.1365-313X.2007.03063.x
Passari AK, Mishra VK, Saikia R et al (2015) Isolation, abundance and phylogenetic affiliation of endophytic actinomycetes associated with medicinal plants and screening for their in vitro antimicrobial biosynthetic potential. Front Microbiol 6:1–13. https://doi.org/10.3389/fmicb.2015.00273
Patrick OR, Abimbola OA, Adeniyi AO (2018) Screening of bacteria isolated from the rhizosphere of maize plant (Zea Mays L.) for ammonia production and nitrogen fixation. Afr J Microbiol Res 12:829–834. https://doi.org/10.5897/ajmr2018.8957
Reimer M, Hartmann TE, Oelofse M et al (2020) Reliance on biological nitrogen fixation depletes soil phosphorus and potassium reserves. Nutr Cycl Agroecosystems 118:273–291. https://doi.org/10.1007/s10705-020-10101-w
Rijavec T, Lapanje A (2016) Hydrogen cyanide in the rhizosphere: not suppressing plant pathogens, but rather regulating availability of phosphate. Front Microbiol 7:1–14. https://doi.org/10.3389/fmicb.2016.01785
Ruiu L (2020) Plant-Growth-Promoting bacteria (PGPB) against insects and other agricultural pests. Agronomy 10:1–12. https://doi.org/10.3390/agronomy10060861
Sachdev DP, Chaudhari HG, Kasture VM et al (2009) Isolation and characterization of Indole acetic acid (IAA) producing Klebsiella pneumoniae strains from rhizosphere of wheat (Triticum aestivum) and their effect on plant growth. Indian J Exp Biol 47:993–1000
Sasirekha B, Shivakumar S, Sullia SB (2012) Statistical optimization for improved indole-3-acetic acid (iaa) production by Pseudomonas aeruginosa and demonstration of enhanced plant growth promotion. J Soil Sci Plant Nutr 12:863–873. https://doi.org/10.4067/s0718-95162012005000038
Singh LS, Sharma H, Talukdar NC (2014) Production of potent antimicrobial agent by actinomycete, streptomyces sannanensis strain SU118 isolated from Phoomdi in Loktak lake of manipur, India. BMC Microbiol 14:1–13. https://doi.org/10.1186/s12866-014-0278-3
Sofo A, Zanella A, Ponge JF (2022) Soil quality and fertility in sustainable agriculture, with a contribution to the biological classification of agricultural soils. Soil Use Manag 38:1085–1112. https://doi.org/10.1111/sum.12702
Spaepen S, Vanderleyden J, Remans R (2007) Indole-3-acetic acid in microbial and microorganism-plant signaling. FEMS Microbiol Rev 31:425–448. https://doi.org/10.1111/j.1574-6976.2007.00072.x
Sreevidya M, Gopalakrishnan S, Kudapa H, Varshney RK (2016) Exploring plant growth-promotion actinomycetes from vermicompost and rhizosphere soil for yield enhancement in Chickpea. Brazilian J Microbiol 47:85–95. https://doi.org/10.1016/j.bjm.2015.11.030
Srinivasan M, Petersen DJ, Holl FB (1996) Influence of indoleacetic acid-producing Bacillus isolates on the nodulation of phaseolus vulgaris by rhizobium Etli under gnotobiotic conditions. Can J Microbiol 42:1006–1014. https://doi.org/10.1139/m96-129
Sun F, Ou Q, Wang N et al (2020) Isolation and identification of potassium-solubilizing bacteria from Mikania Micrantha rhizospheric soil and their effect on M. Micrantha plants. Glob Ecol Conserv 23. https://doi.org/10.1016/j.gecco.2020.e01141
Tian J, Ge F, Zhang D et al (2021) Roles of phosphate solubilizing microorganisms from managing soil phosphorus deficiency to mediating biogeochemical p cycle. Biology (Basel) 10:1–19. https://doi.org/10.3390/biology10020158
Vardharajula S, Shaik ZA (2014) Exopolysaccharide production by drought tolerant Bacillus spp. And effect on soil aggregation under drought stress. J Microbiol Biotechnol Food Sci 4:51–57. https://doi.org/10.15414/jmbfs.2014.4.1.51-57
Wahyudi AT, Priyanto JA, Afrista R et al (2019) Plant growth promoting activity of actinomycetes isolated from soybean rhizosphere. Online J Biol Sci 19:1–8. https://doi.org/10.3844/ojbsci.2019.1.8
Wang CJ, Yang W, Wang C et al (2012) Induction of drought tolerance in cucumber plants by a consortium of three plant Growth-Promoting rhizobacterium strains. PLoS ONE 7:1–10. https://doi.org/10.1371/journal.pone.0052565
Wei G, Kloepper JW, Tuzun S (1991) Induction of systemic resistance of cucumber to Colletotrichum orbiculare by select strains of plant Growth-Promoting rhizobacteria. Phytopathology 81:1508–1512. https://doi.org/10.1094/PHYTO-81-1508
Wiehe W, Schloter M, Hartmann A, Höflich G (1995) Detection of colonization by Pseudomonas PsIA12 of inoculated roots of Lupinus albus and Pisum sativum in greenhouse experiments with immunological techniques. Symbiosis 20:129–145
Xue L, Xue Q, Chen Q et al (2013) Isolation and evaluation of rhizosphere actinomycetes with potential application for biocontrol of verticillium wilt of cotton. Crop Prot 43:231–240. https://doi.org/10.1016/j.cropro.2012.10.002
Xue PP, Carrillo Y, Pino V et al (2018) Soil properties drive microbial community structure in a large scale transect in South Eastern Australia. Sci Rep 8:1–11. https://doi.org/10.1038/s41598-018-30005-8
Yang Y, Zhang S, wen, Li, tai K (2019) Antagonistic activity and mechanism of an isolated streptomyces corchorusii stain AUH-1 against phytopathogenic fungi. World J Microbiol Biotechnol 35:1–9. https://doi.org/10.1007/s11274-019-2720-z
Yushchuk O, Binda E, Marinelli F (2020) Glycopeptide antibiotic resistance genes: distribution and function in the producer actinomycetes. https://doi.org/10.3389/fmicb.2020.01173. Front Microbiol 11:
Zelaya-Molina LX, Guerra-Camacho JE, Ortiz-Alvarez JM et al (2023) Plant growth-promoting and heavy metal-resistant Priestia and Bacillus strains associated with pioneer plants from mine tailings. Arch Microbiol 205:1–23. https://doi.org/10.1007/s00203-023-03650-5
Zhu H, Sandiford SK, Van Wezel GP (2014) Triggers and cues that activate antibiotic production by actinomycetes. J Ind Microbiol Biotechnol 41:371–386. https://doi.org/10.1007/s10295-013-1309-z
Bric JM, Bostock RM, Silverstone SE (1991) Rapid in situ assay for indoleacetic Acid production by bacteria immobilized on a nitrocellulose membrane. Appl Environ Microbiol 157:535–8. https://doi.org/10.1128/aem.57.2.535-538.1991
Damam M, Moinuddin MK, Kausar R (2016) Isolation and screening of plant growth promoting actinomycetes from rhizosphere of some forest medicinal plants. International Journal of Chemtech Reesearch 9:522–528.
Kravchenko LV, Azarova TS, Makarova NM et al (2004) The Effect of Tryptophan Present in Plant Root Exudates on the Phytostimulating Activity of Rhizobacteria. Microbiology 73: 156–158. https://doi.org/10.1023/B:MICI.0000023982.76684.9d
Quiroz-Villareal S, Hernández NZ, Luna-Romero I, Amora-Lazcano E, Rodríguez-Dorantes A (2012) Assessment of plant growth promotion by rhizobacteria supplied with tryptophan as phytohormone production elicitor on Axonopus affinis. Agric Sci Res J 2: 574–580.
Author Information
Laboratory of Agro-Industrial and Medical Biotechnologies, Faculty of Sciences and Technology, University of Sultan Moulay Slimane, Beni-Mellal, Morocco