Prevalance of multifunctional Azospirillum formosense strains in the rhizosphere of pearl millet across diverse edaphoclimatic regions of India
Research Articles | Published: 24 December, 2022
First Page: 1119
Last Page: 1129
Views: 3821
Keywords: Diazotrophs, Colonization, Root traits, Genus specific primers, Diversity
Abstract
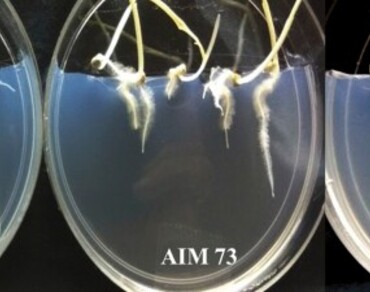
The present study aimed at selecting indigenous Azospirillum spp. strains exhibiting plant growth promoting traits and tolerance to osmotic stress, for their potential application as bioinoculants in rainfed pearl millet. Seventy-five putative Azospirillum spp. isolates were retrieved from the rhizosphere of pearl millet cultivated under diverse edaphoclimatic conditions of India using differential media. The isolates exhibited nitrogenase activity (range between 0.2 and 200 nmoles ethylene produced h− 1), indole acetic acid production (range between 2.5 and 28.3 µg ml− 1), siderophore production and mannitol tolerance (up to 1000 mM). The 16 S rRNA gene sequence (amplified using universal primers 8 F and 1492R) analysis with reference to NCBI nucleotide database revealed that majority of the isolates obtained from different locations exhibited maximum identity (93.31–99.32%) to A. formosense, whereas two isolates AIM1 and AIM45 exhibited maximum identity to A. soli (98.54% identity) and A. oryzae (93.86% identity), respectively. However, the homology below 97% indicated the possibility of novel strains/species among the isolates. Selected strains were evaluated by designing a vertical agar plate assembly (VAPA) for their effect on root traits and growth of pearl millet (RHB173) seedlings. Bacterial inoculation significantly improved plant biomass (shoot and root) and influenced root architecture (root branching and root hair density). Microscopic observations revealed extensive colonization on the root surface as well as in the endorhizosphere. The VAPA assay helped in selecting promising strains AIM19, AIM57, AIM80, AIM38 and AIM3 which were able to influence plant growth and root architecture, the trait helpful in survival under stressful conditions. The present study indicated the abundance of multifunctional A. formosense strains in the rhizosphere of pearl millet grown under diverse locations. Also, the VAPA assay demonstrated in the present study that it can be used for the screening of large number of isolates for early stage plant growth promotion studies.
References
Aleksandrov VG, Blagodyr RN, Ilev IP (1967) Liberation of phosphoric acid from apatite by silicate bacteria. Mikrobiol Zhurnal (Kiev) 29:111–114
Alexander M (1965) Methods of soil analysis, part 2. Agronomy series #9. ASA, Madison, pp 1467–1472
Baldani JI, Reis VM, Videira SS, Boddey LH, Baldani VLD (2014) The art of isolating nitrogen-fixing bacteria from non-leguminous plants using N-free semi-solid media: a practical guide for microbiologists. Plant Soil 384:413–431. doi:https://doi.org/10.1007/s11104-014-2186-6
Bardgett RD, Mommer L, De Vries FT (2014) Going underground: root traits as drivers of ecosystem processes. Trends Ecol Evol 29(12):692–699. https://doi.org/10.1016/j.tree.2014.10.006
Bashan Y, de-Bashan LE (2010) How the plant growth-promoting bacterium Azospirillum promotes plant growth: a critical assessment. Adv Agron 108:77–136. https://doi.org/10.1016/S0065-2113(10)08002-8
Cassán F, Coniglio A, López G, Molina R, Nievas S, de Carlan CLN et al (2020) Everything you must know about Azospirillum and its impact on agriculture and beyond. Biol Fertil Soils 56(4):461–479. doi:https://doi.org/10.1007/s00374-020-01463-y
Chowdhury SP, Nagarajan T, Tripathi R, Mishra MN, Le Rudulier D, Tripathi AK (2007) Strain-specific salt tolerance and osmoregulatory mechanisms in Azospirillum brasilense. FEMS Microbiol Lett 267(1):72–79. https://doi.org/10.1111/j.1574-6968.2006.00540.x
Döbereiner J (1995) Isolation and identification of aerobic nitrogen-fixing bacteria from soil and plants. In: Alef K, Nannipieri P (eds) Methods in applied soil microbiology and biochemistry. Academic Press, London, pp 134–141
Döbereiner J, Day JM (1976) Associative symbioses in tropical grasses: characterization of microorganisms and dinitrogen-fixing sites. In: Newton WE, Nyman CJ (eds) Proceedings of the first international symposium on nitrogen fixation. Washington State University Press, Pullman, pp 518–538
Evseeva NV, Tkachenko OV, Denisova AY, Burygin GL, Veselov DS, Matora LY, Shchyogolev SY (2019) Functioning of plant-bacterial associations under osmotic stress in vitro. World J Microb Biot 35(12):195. doi:https://doi.org/10.1007/s11274-019-2778-7
Ferreira AS, Pires RR, Rabelo PG, Oliveira RC, Luz JMQ, Brito CH (2013) Implications of Azospirillum brasilense inoculation and nutrient addition on maize in soils of the brazilian cerrado under greenhouse and field conditions. App Soil Ecol 72:103–108. https://doi.org/10.1016/j.apsoil.2013.05.020
Fujita M, Kusajima M, Okumura Y, Nakajima M, Minamisawa K, Nakashita H (2017) Effects of colonization of a bacterial endophyte, Azospirillum sp. B510, on disease resistance in tomato. Biosci Biotechnol Biochem 81(8):1657–1662. doi:https://doi.org/10.1080/09168451.2017.1329621
Fukami J, Cerezini P, Hungria M (2018) Azospirillum: benefits that go far beyond biological nitrogen fixation. AMB Expr 8:73. https://doi.org/10.1186/s13568-018-0608-1
Galindo FS, Teixeira Filho MCM, Buzetti S, Pagliari PH, Santini JMK, Alves CJ, Megda MM, Nogueira TAR, Andreotti M, Arf O (2019) Maize yield response to nitrogen rates and sources associated with Azospirillum brasilense. Agron J 111(4):1985–1997. https://doi.org/10.2134/agronj2018.07.0481
García JE, Maroniche G, Creus C, Suárez-Rodríguez R, Ramirez-Trujillo JA, Groppa MD (2017) In vitro PGPR properties and osmotic tolerance of different Azospirillum native strains and their effects on growth of maize under drought stress. Microbiol Res 202:21–29. https://doi.org/10.1016/j.micres.2017.04.007
Gordon SA, Weber RP (1951) Colorimetric estimation of indoleacetic acid. Plant Physiol 26:192–195. https://doi.org/10.1104/pp.26.1.192
Grover M, Ali SKZ, Sandhya V, Rasul A, Venkateswarlu B (2011) Role of microorganisms in adaptation of agriculture crops to abiotic stress. World J Microbiol Biotechnol 27:1231–1240. https://doi.org/10.1007/s11274-010-0572-7
Hardy RWF, Holsten RD, Jackson EK, Burns RC (1968) The acetylene-ethylene assay for N2 fixation: laboratory and field evaluation. Plant Physiol 43:1185–1207. https://dx.doi.org/10.1104%2Fpp.43.8.1185
Hartmann A, Singh M, Klingmüller W (1983) Isolation and characterization of Azospirillum mutants excreting high amounts of indoleacetic acid. Can J Microbiol 29:916–923. https://doi.org/10.1139/m83-147
Krieg NR, Dobereiner J (1984) Bergey’s manual of systematic bacteriology. In: Krieg NR, Holt JG (eds) The genus Azospirillum, 1st edn. The Williams and Wilkins Co, Baltimore, pp 194–104
Leite RC, dos Santos JGD, Silva EL, Alves CRCR, Hungria M, Leite RC, dos Santos AC (2019) Productivity increase, reduction of nitrogen fertiliser use and drought-stress mitigation by inoculation of Marandu grass (Urochloa brizantha) with Azospirillum brasilense. Crop Pasture Sci 70:61–67. https://doi.org/10.1071/CP18105
Lin SY, Shen FT, Young CC (2011) Rapid detection and identification of the free-living nitrogen fixing genus Azospirillum by 16S rRNA-gene-targeted genus-specific primers. Antonie Van Leeuwenhoek 99:837–844. https://doi.org/10.1007/s10482-011-9558-1
Maroniche GA, García JE, Salcedo F, Creus CM (2017) Molecular identification of Azospirillum spp.: limitations of 16S rRNA and qualities of rpoD as genetic markers. Microbiol Res 195:1–10. https://doi.org/10.1016/j.micres.2016.11.009
Miethke M, Marahiel MA (2007) Siderophore-based iron acquisition and pathogen control. Microbiol Mol Biol Rev 71(3):413–451. https://dx.doi.org/10.1128%2FMMBR.00012-07
Okon Y (1982) Azospirillum: physiological properties, mode of association with roots and its application for the benefit of cereal and forage grass crops. Isr J Bot 31:214–220. https://doi.org/10.1080/0021213X.1982.10676945
Okon Y, Labandera-Gonzalez CA (1994) Agronomic applications of Azospirillum: an evaluation of 20 years worldwide field inoculation. Soil Biol Biochem 26(12):1591–1601. https://doi.org/10.1016/0038-0717(94)90311-5
Pandiarajan G, Kumar NT BM (2012) Exploration of different Azospirillum strains from various crop soils of srivilliputtur taluk. J Biofertil Biopestici 3:117. DOI:https://doi.org/10.4172/2155-6202.1000117
Pereg L, de-Bashan LE, Bashan Y (2016) Assessment of affinity and specificity of Azospirillum for plants. Plant Soil 399:389–414. https://doi.org/10.1007/s11104-015-2778-9
Pikovskaya RI (1948) Mobilization of phosphorus and soil in connection with the vital activity of some microbial species. Mikrobiologia 17:362–370
Ribaudo CM, Krumpholz EM, Cassán FD, Bottini R, Cantore ML, Curá JA (2006) Azospirillum sp. promotes root hair development in tomato plants through a mechanism that involves ethylene. J Plant Growth Regul 25:175–185. https://doi.org/10.1007/s00344-005-0128-5
Rodrignuez-Cáceres EA (1982) Improved medium for isolation of Azospirillum spp. Appl Environ Microbiol 44(4):990–991
Saikia SP, Bora D, Goswami A, Gogoi A (2012) A review on the role of Azospirillum in the yield improvement of non-leguminous crops. Afr J Microbiol Res 6(6):1085–1102. DOI: https://doi.org/10.5897/AJMR11.019
Saitou N, Nei M (1987) The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol 4:406–425. https://doi.org/10.1093/oxfordjournals.molbev.a040454
Sambrook J, Fritsch EF, Maniatis T (1989) Molecular cloning. A laboratory manual, 2nd edn. Cold Spring Harbor laboratory, NewYork
Santana SRA, Voltolini TV, Antunes GR dos, da Silva VM, Simões WL, Morgante CV, Santiago de Freitas AD, Chaves ARM, Aidar ST, Fernandes Júnior PI (2020) Inoculation of plant growth-promoting bacteria attenuates the negative effects of drought on sorghum. Arch Microbiol 202:1015–1024. https://doi.org/10.1007/s00203-020-01810-5
Saravanan VS, Subramoniam SR, Raj SA (2004) Assessing in vitro solubilization potential of different zinc solubilizing bacterial (ZSB) isolates. Braz J Microbiol 35(1–2):121–125. https://doi.org/10.1590/S1517-83822004000100020
Schwyn B, Neilands JB (1987) Universal chemical assay for the detection and determination of siderophore. Anal Biochem 160:47–56. https://doi.org/10.1016/0003-2697(87)90612-9
Sutaliya R (2019) Performance of pearl millet advance hybrids to different levels of nitrogen under dry land conditions. Int J Curr Microbiol Appl Sci 8(07):2245–2248. https://doi.org/10.20546/ijcmas.2019.807.273
Swedrzynska D, Sawicka A (2001) Effect of inoculation on population numbers of Azospirillum bacteria under winter wheat, oat and maize. Pol J Environ Stud 10(1):21–25
Tamura K, Stecher G, Peterson D, Filipski A, Kumar S (2013) MEGA6: molecular evolutionary genetics analysis version 6.0. Mol Biol Evol 30(12):2725–2729. https://doi.org/10.1093/molbev/mst197
Tiwari M, Paroda S, Dadarwal NR (2003) Associative diazotrophs of pearl millet (Pennisetum glaucum) from semiarid region: isolation and characterization. Indian J Exp Biol 41:341–345
Varshney R, Shi C, Thudi M, Mariac C, Wallace J, Qi P et al (2017) Pearl millet genome sequence provides a resource to improve agronomic traits in arid environments. Nat Biotechnol 35:969–976. https://doi.org/10.1038/nbt.3943
Verma R, Chourasia SK, Jha MN (2011) Population dynamics and identification of efficient strains of Azospirillum in maize ecosystems of Bihar (India). 3Biotech 1(4):247–253. https://doi.org/10.1007/s13205-011-0031-7
Zeffa DM, Perini LJ, Silva MB, de Souza NV, Scapim CA, de Oliveira ALM et al (2019) Azospirillum brasilense promotes increases in growth and nitrogen use efficiency of maize genotypes. PLoS One 14(4):e0215332
LPSN (List of Prokaryotic Names with Standing in Nomenclature (2022) https://lpsn.dsmz.de/search?word=azospirillum Accessed on 29
Tara Satyavathi C, Praveen S, Saikat M, Chugh LK, Kawatra A (2017) Enhancing demand of pearl millet as super grain - current status and way forward. ICAR-All India Coordinated Research Project on Pearl millet, Jodhpur-342304 pp 1–20. ISBN:978-93-5279-718-9
Author Information
Division of Microbiology, Indian Agricultural Research Institute, New Delhi, India