Production of bioethanol from biodegraded alkali pretreated rice straw
Research Articles | Published: 02 January, 2020
First Page: 128
Last Page: 134
Views: 3953
Keywords: T. reesei , S. cerevisiae , Bioethanol, Rice straw
Abstract
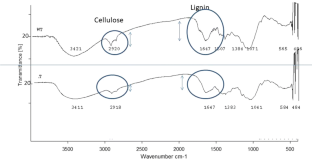
NaOH (1%) treated paddy straw was neutralized for pH with 10% HCl. The result of pre-treatment on straw was examined by Fourier-transform infrared spectroscopy (FTIR) analysis. FTIR spectra revealed the degradation of straw cellulose and lignin. The broadband observation 3421 of untreated and 3411 cm−1 of pre-treated straw shows O–H stretching hydrogen bond, 2920 is C–H stretching showing changes during NaOH pre-treatment. Similarly peaks around 1600 cm−1 is assigned to lignin and this peak was reduced in pretreated straw. The pre-treated straw was further biodegraded by inoculation of Trichoderma reesei (treatments were; T1—pre-treated straw + T. reesei, T2—rice + T. reesei, T3—straw, T4—sterilized straw + T. reesei, T5—pre-treated sterilized straw + T. reesei). Results revealed that best treatment was T5 followed by T2 and T4. In case of treatment T5 i.e. pre-treated sterilized straw + T. reesei the FTIR graphs was approximately flat showing a complete degradation of paddy straw. Peaks from 2400 to 900 cm−1 were missing in treatment T5 whereas rest of the peaks for lignin (1600–1515 cm−1), Hemicelluluse (2400 cm−1) and cellulose (3400–3020 cm−1, 1215–1025 cm−1) were present in other treatments. To detect bioethanol production Saccharomyces cerevisiae was inoculated into treatment T4 and T5. The ethanol production was confirmed by GC–MS in treated and untreated straw after yeast inoculation.
References
- Belal EB (2013) Bioethanol production from rice straw residues. Braz J Microbiol 44(1):225–234
- Bhat MK, Bhat S (1997) Cellulose degrading enzymes and their potential industrial applications. Biotechnol Adv 15(3–4):583–620
- Binod P, Satyanagalakshmi K, Sindhu R, Janu KU, Sukumaran RK, Pandey A (2012) Short duration microwave assisted pretreatment enhances the enzymatic saccharification and fermentable sugar yield from sugarcane bagasse. Renew Energy 37:109–116
- Bradner JR, Gillings M, Nevalainen KMH (1999) Qualitative assessment of hydrolytic activities in antarctic microfungi grown at different temperatures on solid media. World J Microbiol Biotechnol 15(143):145
- Cherubini F, Ulgiati S (2010) Crop residues as raw materials for biorefinery systems—a LCA case study. Appl Energy 87:47–57
- Choi IS, Kim JH, Wi SG, Kim KH, Bae H-J (2012) Bioethanol production from mandarin (Citrus unshiu) peel waste using popping pretreatment. Appl Energy 102:204–210
- Cuevas VC (1997) Rapid composting technology in the Philippines: Its role in producing good-quality organic fertilizers. Food Fertilizer Technology. Cent Extn Bull 444:1–13
- Dobermann A, Fairhurst TH (2002) Rice straw management. Better crops. International 16(Sp. Supp. May):7–9
- Galbe M, Zacchi G (2002) A review of the production of ethanol from softwood. Appl Microbiol Biotechnol 59:618–628
- Jahangeer S, Khan N, Jahangeer S, Sohail M, Shahzad S, Ahmad A, Khan SA (2005) Screening and characterization of fungal cellulases isolated from the native environmental source. Pak J Bot 37:739–748
- Jin S, Chen H (2007) Fractionation of fibrous fraction from steam-exploded rice straw. Process Biochem 42:188–192
- Liu CF, Xu F, Sun JX, Ren JL, Curling S, Sun RC, Fowler P, Baird MS (2006) Physicochemical characterisation of cellulose from perennial ryegrass leaves (Lolium perenne). Carbohydr Res 341:2677–2687
- Lun, LW, Gunny, Kasim AAN, Arbain FH (2017) In Fourier transform infrared spectroscopy (FTIR) analysis of paddy straw pulp treated using deep eutectic solvent. In: Proceedings of AIP conference, Kaohsiung City, Taiwan, Dec 89, 2016
- Matsumura Y, Minowa T, Yamamoto H (2005) Amount, availability, and potential use of rice straw (agricultural residue) biomass as an energy resource in Japan. Biomass Bioenergy 29:347–354
- Matthews S (2016) Structural changes of rice straw pre-treated with Paenibacillus and Aspergillus fumigatus. Int J Agric Food Res 5:1–8
- Miller G (1959) Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem 31:426–428
- Momayez F, Karimi K, Karimi S, Horváth IS (2017) Efficient hydrolysis and ethanol production from rice straw by pretreatment with organic acids and effluent of biogas plant. R Soc Chem 7:50537–50545
- Ortega N, Busto MD, Perez-Mateos M (2001) Kinetics of cellulose saccharification by Trichoderma reseeicellulases. Int Biodeterior Biodegrad 47:7–14
- Saha BC (2003) Hemicellulose bioconversion. J Ind Microbiol Biotechnol 30:279–291
- Sun Y, Cheng J (2002) Hydrolysis of lignocellulosic materials for ethanol production: a review. Bioresour Technol 83(1):1–11
- Walker GM, Stewart GG (2016) Saccharomyces cerevisiae in the production of fermented beverages. Beverages 2:30
Author Information
Department of Agricultural Microbiology, College of Agriculture, Raipur, India