Rhizosphere metagenomics of wild and cultivated Caesalpinia bonducella L. depict similarities in their microflora
Research Articles | Published: 01 September, 2022
First Page: 877
Last Page: 889
Views: 4675
Keywords: Caesalpinia bonducella , Microbial diversity, Oxford nanopore technology, Poly cystic ovary syndrome, Rhizosphere–rhizomicrobiome
Abstract
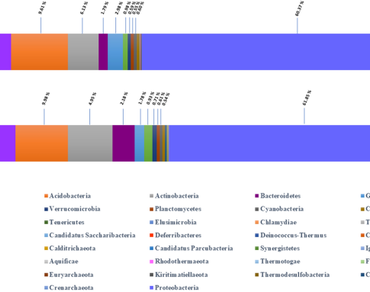
Exploring the rhizomicrobiome and its functional traits associated with the plant assists in enhancing the plant’s metabolites for its application in medicine. This study identified the rhizomicrobiome of wild and cultivated Caesalpinia bonducella, a potential medicinal plant used for the treatment of polycystic ovary syndrome. The microflora between the plants was assumed to vary because of the varied endurances in different culture environments. For this, soil microbial DNA was extracted from the rhizosphere regions and the 16S rDNA was sequenced using oxford nanopore technology. The results showed that the rhizomicrobiome did not vary significantly between wild and cultivated plant. The alpha diversity indices represented highly diverse bacterial communities primarily belonging to the phyla Proteobacteria, Actinobacteria, Acidobacteria and Firmicutes representing 45,018 and 48,949 operational taxonomic units in the wild and cultivated plant respectively. Furthermore, the bacterial communities categorized based on its functions could help in domestication of this plant with enhanced metabolites.
References
Ait Kaki A, Kacem Chaouche N, Dehimat L, Milet A, Youcef-Ali M, Ongena M, Thonart P (2013) Biocontrol and plant growth promotion characterization of Bacillus species isolated from Calendula officinalis rhizosphere. Indian J Microbiol 53:447–452. https://doi.org/10.1007/s12088-013-0395-y
Akinsanya MA, Goh JK, Lim SP, Ting ASY (2015) Metagenomics study of endophytic bacteria in Aloe vera using next-generation technology. Genomics Data 6:159–163. https://doi.org/10.1016/j.gdata.2015.09.004
Alexander A, Singh VK, Mishra A, Jha B (2019) Plant growth promoting rhizobacterium Stenotrophomonas maltophilia BJ01 augments endurance against N2 starvation by modulating physiology and biochemical activities of Arachis hypogea. PLoS ONE 14:9. https://doi.org/10.1371/journal.pone.0222405
Badri DV, Vivanco JM (2009) Regulation and function of root exudates. Plant Cell Environ 32:666–681. https://doi.org/10.1111/j.1365-3040.2009.01926.x
Bafana A (2013) Diversity and metabolic potential of culturable root-associated bacteria from Origanum vulgare in sub-Himalayan region. World J Microbiol Biotechnol 29:63–74. https://doi.org/10.1007/s11274-012-1158-3
Bahadur A, Singh UP, Sarma BK, Singh DP, Singh KP, Singh A (2007) Foliar Application of Plant growth-promoting rhizobacteria increases antifungal compounds in pea (Pisum sativum) against Erysiphe pisi. Mycobiology 35:129. https://doi.org/10.4489/myco.2007.35.3.129
Banchio E, Bogino PC, Zygadlo J, Giordano W (2008) Plant growth promoting rhizobacteria improve growth and essential oil yield in Origanum majorana L. Biochem Syst Ecol 36:766–771. https://doi.org/10.1016/j.bse.2008.08.006
Banchio E, Xie X, Zhang H, Paré PW (2009) Soil bacteria elevate essential oil accumulation and emissions in sweet basil. J Agric Food Chem 57:653–657. https://doi.org/10.1021/jf8020305
Banchio E, Bogino PC, Santoro M, Torres L, Zygadlo J, Giordano W (2010) Systemic induction of monoterpene biosynthesis in Origanum majoricum by soil bacteria. J Agric Food Chem 13:650–654. https://doi.org/10.1021/jf9030629
Bellini MI, Gutiérrez L, Tarlera S, Scavino AF (2013) Isolation and functional analysis of denitrifiers in an aquifer with high potential for denitrification. Syst Appl Microbiol 36:505–516. https://doi.org/10.1016/j.syapm.2013.07.001
Bharti N, Yadav D, Barnawal D, Maji D, Kalra A (2013) Exiguobacterium oxidotolerans, a halotolerant plant growth promoting rhizobacteria, improves yield and content of secondary metabolites in Bacopa monnieri (L.) Pennell under primary and secondary salt stress. World J Microbiol Biotechnol 29:379–387. https://doi.org/10.1007/s11274-012-1192-1
Bharti N, Barnawal D, Awasthi A, Yadav A, Kalra A (2014) Plant growth promoting rhizobacteria alleviate salinity induced negative effects on growth, oil content and physiological status in Mentha Arvensis. Acta Physiol Plant 36:45–60. https://doi.org/10.1007/s11738-013-1385-8
Breidenbach B, Pump J, Dumont MG (2016) Microbial community structure in the rhizosphere of rice plants. Front Microbiol. https://doi.org/10.3389/fmicb.2015.01537
Cappellari L del R, Santoro MV, Nievas F, Giordano W, Banchio E (2013) Increase of secondary metabolite content in marigold by inoculation with plant growth-promoting rhizobacteria. Appl Soil Ecol 70:16–22. https://doi.org/10.1016/j.apsoil.2013.04.001
Chen H, Wu H, Yan B, Zhao H, Liu F, Zhang H, Liang Z (2018) Core microbiome of medicinal plant salvia miltiorrhiza seed: a rich reservoir of beneficial microbes for secondary metabolism? Int J Mol Sci 19:3. https://doi.org/10.3390/ijms19030672
Chukwuneme CF, Babalola OO, Kutu FR, Ojuederie OB (2020) Characterization of actinomycetes isolates for plant growth promoting traits and their effects on drought tolerance in maize. J Plant Interact 15:93–105. https://doi.org/10.1080/17429145.2020.1752833
Egamberdieva D, Jabborova D, Mamadalieva N (2013) Salt tolerant Pseudomonas extremorientalis able to stimulate growth of Silybum marianum under salt stress. Med Aromatic Plant Sci Biotechnol 7:7–10
Enagbonma BJ, Aremu BR, Babalola OO (2019) Profiling the functional diversity of termite mound soil bacteria as revealed by shotgun sequencing. Genes 10:9. https://doi.org/10.3390/genes10090637
Ghorbanpour M, Hatami M, Khavazi K (2013a) Role of plant growth promoting rhizobacteria on antioxidant enzyme activities and tropane alkaloid production of Hyoscyamus niger under water deficit stress. Turk J Biol 37:350–360. https://doi.org/10.3906/biy-1209-12
Ghorbanpour M, Ghafarzadegan R, Khavazi K, Hatami M (2013b) Two main tropane alkaloids variations of black henbane (hyoscyamus niger) under PGPRs inoculation and water deficit stress induction at flowering stage. J Med Plants 12:29–42
Hanson CA, Fuhrman JA, Horner-Devine MC, Martiny JB (2012) Beyond biogeographic patterns: processes shaping the microbial landscape. Nat Rev Microbiol 10:497–506. https://doi.org/10.1038/nrmicro2795
Heidari M, Golpayegani A (2012) Effects of water stress and inoculation with plant growth promoting rhizobacteria (PGPR) on antioxidant status and photosynthetic pigments in basil (Ocimum basilicum L.). J Saudi Soc Agric Sci 11:57–61. https://doi.org/10.1016/j.jssas.2011.09.001
Hemashenpagam N, Selvaraj T (2011) Effect of arbuscular mycorrhizal (AM) fungus and plant growth promoting rhizomicroorganisms (PGPR’s) on medicinal plant Solanum viarum seedlings. J Environ Biol 32:579–583
Jacoby R, Peukert M, Succurro A, Koprivova A, Kopriva S (2017) The role of soil microorganisms in plant mineral nutrition—current knowledge and future directions. Front Plant Sci. https://doi.org/10.3389/fpls.2017.01617
Jaleel CA, Gopi R, Gomathinayagam M, Panneerselvam R (2009) Traditional and non-traditional plant growth regulators alters phytochemical constituents in Catharanthus roseus. Process Biochem 44:205–209. https://doi.org/10.1016/j.procbio.2008.10.012
Janja F, Ioanna V, Elena S, Francesco C, Sanda S, Nicola U, Ivano V, Patricija M, Živana NG, As G, Alexandra P, Soultana Z, Kalliopi P, Alberto B (2021) Large-scale testing of phytoplankton diversity indices for environmental assessment in Mediterranean sub-regions (Adriatic, Ionian and Aegean Seas). Ecol Ind 126:470–160. https://doi.org/10.1016/j.ecolind.2021.107630
Khamna S, Yokota A, Peberdy JF, Lumyong S (2010) Indole-3-acetic acid production by Streptomyces sp. isolated from some Thai medicinal plant rhizosphere soils. EurAsian J Biosci. https://doi.org/10.5053/ejobios.2010.4.0.4
Kim MJ, Radhakrishnan R, Kang SM, You YH, Jeong EJ, Kim JG, Lee IJ (2017) Plant growth promoting effect of Bacillus amyloliquefaciens H-2-5 on crop plants and influence on physiological changes in soybean under soil salinity. Physiol Mol Biol Plants 23:571–580. https://doi.org/10.5053/ejobios.2010.4.0.4
Köberl M, Schmidt R, Ramadan EM, Bauer R, Berg G (2013) The microbiome of medicinal plants: diversity and importance for plant growth, quality, and health. Front Microbiol 4:400. https://doi.org/10.3389/fmicb.2013.00400
Kumar A, Soni R, Kanwar SS (2019) Stenotrophomonas: a versatile diazotrophic bacteria from the rhizospheric soils of Western Himalayas and development of its liquid biofertilizer formulation. Vegetos 32:103–109. https://doi.org/10.1007/s42535-019-00013-8
Lenin G, Jayanthi M (2012) Efficiency of plant growth promoting rhizobacteria (PGPR) on enhancement of growth, yield and nutrient content of Catharanthus roseus. Int J Res Pure Appl Microbiol 2:37–42. https://doi.org/10.1088/1755-1315/166/1/012022
Lilaram, Nazeer Ahamed R (2013) Effect of Caesalpinia bonducella seed extract on histoarchitecture of some vital organs and clinical chemistry in female albino rats. J King Saud Univ Sci 25:1–6. https://doi.org/10.1016/j.jksus.2012.09.001
Luo Y, Wang F, Huang Y, Zhou M, Gao J, Yan T, An L (2019) Sphingomonas sp. Cra20 increases plant growth rate and alters rhizosphere microbial community structure of Arabidopsis thaliana under drought stress. Front Microbiol. https://doi.org/10.3389/fmicb.2019.01221
Mansoor F, Sultana V, Ehteshamul-Haque S (2007) Enhancement of biocontrol potential of pseudomonas aeruginosa and paecilomyces lilacinus against root rot of mungbean by a medicinal plant Launaea nudicaulis L. Pak J Bot 39:2113–2119
Meera B, Muralidharan P, Hari R (2020) Antioxidant potential of Caesalpenia bonducella seeds in the management of polycystic ovary syndrome (PCOS) using mifepristone induced rats model. J Herbs Spices Med Plants. https://doi.org/10.1080/10496475.2020.1795041
Mishra M, Kumar U, Mishra PK, Prakash V (2010) Efficiency of plant growth promoting rhizobacteria for the enhancement of Cicer arietinum L. growth and germination under salinity. Adv Biol Res 4:92–96
Mitter B, Brader G, Afzal M, Compant S, Naveed M, Trognitz F, Sessitsch A (2013) Advances in elucidating beneficial interactions between plants, soil, and bacteria. In: Advances in agronomy (Academic Press Inc.), pp 381–445. https://doi.org/10.1016/B978-0-12-407685-3.00007-4
Mohanram S, Kumar P (2019) Rhizosphere microbiome: revisiting the synergy of plant–microbe interactions. Ann Microbiol 69:307–320. https://doi.org/10.1007/s13213-019-01448-9
Mohanty P, Singh PK, Chakraborty D, Mishra S, Pattnaik R (2021) Insight into the role of PGPR in sustainable agriculture and environment. Front Sustain Food Syst. https://doi.org/10.3389/fsufs.2021.667150
Mukerji KG, Manoharachary C, Singh J (2006) Microbial activity in the rhizosphere. Springer, Germany, p 349
Namdeo A, Patil S, Fulzele DP (2002) Influence of fungal elicitors on production of ajmalicine by cell cultures of catharanthus roseus. Biotechnol Prog 18:159–162. https://doi.org/10.1021/bp0101280
Pagnani G, Pellegrini M, Galieni A, D’Egidio S, Matteucci F, Ricci A (2018) Plant growth-promoting rhizobacteria (PGPR) in cannabis sativa ‘finola’ cultivation: an alternative fertilization strategy to improve plant growth and quality characteristics. Ind Crops Prod 123:75–83. https://doi.org/10.1016/j.indcrop.2018.06.033
Pankaj U, Singh DN, Singh G, Verma RK (2019) Microbial inoculants assisted growth of chrysopogon zizanioides promotes phytoremediation of salt affected soil. Indian J Microbiol 59:137–146. https://doi.org/10.1007/s12088-018-00776-9
Paytuví A, Battista E, Scippacercola F, Aiese Cigliano R, Sanseverino W (2019) GAIA: an integrated metagenomics suite. BioRxiv. https://doi.org/10.1101/804690
Pedone-Bonfim MVL, da Silva FSB, Maia LC (2015) Production of secondary metabolites by mycorrhizal plants with medicinal or nutritional potential. Acta Physiol Plant. https://doi.org/10.1007/s11738-015-1781-3
Prasad G, Trimurtulu G, Reddy K, Naidu M (2010) Analytical study of Kuberaksha/Kantaki Karanja Patra Churna [Caesalpinia Bonduc (L.) Roxb. leaf powder]. AYU (An International Quarterly Journal of Research in Ayurveda) 31:251. https://doi.org/10.4103/0974-8520.72410
Qi X, Wang E, Chen X (2013) Molecular characterization of bacterial population in the Rumex Patientia Rhizosphere soil of Jilin, China. Res J Biotechnol 8:64–71
Raichand R, Kaur I, Singh NK, Mayilraj S (2011) Pontibacter rhizosphera sp. nov., isolated from rhizosphere soil of an Indian medicinal plant Nerium indicum. Antonie Van Leeuwenhoek Int J General Mol Microbiol 100:129–135. https://doi.org/10.1007/s10482-011-9573-2
Rajkumar M, Bruno LB, Banu JR (2017) Alleviation of environmental stress in plants: the role of beneficial Pseudomonas spp. Crit Rev Environ Sci Technol 47:372–407. https://doi.org/10.1080/10643389.2017.1318619
Ramakrishna W, Yadav R, Li K (2019) Plant growth promoting bacteria in agriculture: two sides of a coin. Appl Soil Ecol 138:10–18. https://doi.org/10.1016/j.apsoil.2019.02.019
Ramyasmruthi S, Pallavi O, Pallavi S, Tilak K, Srividya S (2012) Chitinolytic and secondary metabolite producing Pseudomonas fluorescens isolated from Solanaceae rhizosphere effective against broad spectrum fungal phytopathogens. Pelagia Res Library Asian J Plant Sci Res 2:1–9
Ranjan R, Rani A, Metwally A, McGee HS, Perkins DL (2016) Analysis of the microbiome: Advantages of whole genome shotgun versus 16S amplicon sequencing. Biochem Biophys Res Commun 469:967–977. https://doi.org/10.1016/j.bbrc.2015.12.083
Ray T, Pandey SS, Pandey A, Srivastava M, Shanker K, Kalra A (2019) Endophytic consortium with diverse gene-regulating capabilities of benzylisoquinoline alkaloids biosynthetic pathway can enhance endogenous morphine biosynthesis in Papaver somniferum. Front Microbiol 10:925. https://doi.org/10.3389/fmicb.2019.00925
Rekha K, Baskar B, Srinath S, Usha B (2018) Plant-growth-promoting rhizobacteria Bacillus subtilis RR4 isolated from rice rhizosphere induces malic acid biosynthesis in rice roots. Can J Microbiol 64:20–27. https://doi.org/10.1139/cjm-2017-0409
Rekha K, Ramasamy M, Rameshthangam P, Usha B (2019) Impact of Pseudomonas Putida RRF3 on the root transcriptome of Rice plants: insights into defense response, secondary metabolism and root exudation. J Biosci 44:4. https://doi.org/10.1007/s12038-019-9922-2
Rekha K, Ramasamy M, Usha B (2020) Root exudation of organic acids as affected by plant growth-promoting rhizobacteria Bacillus subtilis RR4 in rice. J Crop Improv 34:571–586. https://doi.org/10.1080/15427528.2020.1746719
Risvan MY, Suresh S, Balagurusamy K (2017) Siddha elixir and aeitology of polycystic ovarian syndrome. Adv Techn Biol Med. https://doi.org/10.4172/2379-1764.1000249
Salunke KR, Ahmed RN, Lilaram SRM (2011) Effect of graded doses of Caesalpinia bonducella seed extract on ovary and uterus in albino rats. J Basic Clin Physiol Pharmacol 22:49–53. https://doi.org/10.1515/JBCPP.2011.006
Santoro MV, Zygadlo J, Giordano W, Banchio E (2011) Volatile organic compounds from rhizobacteria increase biosynthesis of essential oils and growth parameters in peppermint (Mentha piperita). Plant Physiol Biochem 49:1177–1182. https://doi.org/10.1016/j.plaphy.2011.07.016
Shah JA, Pandit AK (2013) Application of diversity indices to crustacean community of Wular Lake, Kashmir Himalaya. Int J Biodivers Conserv 5:311–316
Shi JY, Yuan XF, Lin HR, Yang YQ, Li ZY (2011) Differences in soil properties and bacterial communities between the rhizosphere and bulk soil and among different production areas of the medicinal plant Fritillaria thunbergii. Int J Mol Sci 12:3770–3785. https://doi.org/10.3390/ijms12063770
Shi S, Chang J, Tian L, Nasir F, Ji L, Li X, Tian C (2019) Comparative analysis of the rhizomicrobiome of the wild versus cultivated crop: insights from rice and soybean. Arch Microbiol 201:879–888. https://doi.org/10.1007/s00203-019-01638-8
Sood G, Kaushal R, Sharma M (2020) Significance of inoculation with Bacillus subtilis to alleviate drought stress in wheat (Triticum aestivum L.). Vegetos 33:782–792. https://doi.org/10.1007/s42535-020-00149-y
Srivastava R, Srivastava AK, Ramteke PW, Gupta VK, Srivastava AK (2020) Metagenome dataset of wheat rhizosphere from Ghazipur region of Eastern Uttar Pradesh. Data Brief 28:105095. https://doi.org/10.1016/j.dib.2019.105094
Taghinasab M, Jabaji S (2020) Cannabis microbiome and the role of endophytes in modulating the production of secondary metabolites: an overview. Microorganisms 8:355. https://doi.org/10.3390/microorganisms8030355
Tahir HAS, Gu Q, Wu H, Raza W, Hanif A, Wu L, Gao X (2017) Plant growth promotion by volatile organic compounds produced by Bacillus subtilis SYST2. Front Microbiol. https://doi.org/10.3389/fmicb.2017.00171
Tilak K, Ranganayaki N, Pal KK, De R, Saxena AK, Nautiyal CS, Mittal S, Tripathi AK, Johri BN (2005) Diversity of plant growth and soil health supporting bacteria. Curr Sci 89:136–150
Veerapandiyan K, Usha B (2021) Caesalpinia bonduc (L.) Roxb. as a promising source of pharmacological compounds to treat Poly Cystic Ovary Syndrome (PCOS): a review. J Ethnopharmacol 279:0378–8741. https://doi.org/10.1016/j.jep.2021.114375
Vidya Bharathi R, Swetha S, Neerajaa J, Varsha Madhavica J, Janani DM, Rekha SN, Usha B (2017) An epidemiological survey: effect of predisposing factors for PCOS in Indian urban and rural population. Middle East Fertil Soc J 22:313–316. https://doi.org/10.1016/j.mefs.2017.05.007
Wang X, Guo X, Hou L, Zhang J, Hu J, Zhang F, Shan C (2022) A comparative study of bacterial diversity based on effects of three different shade shed types in the rhizosphere of Panax quiquefolium L. Peer J. https://doi.org/10.7717/peerj.12807
War Nongkhlaw FM, Joshi SR (2014) Epiphytic and endophytic bacteria that promote growth of ethnomedicinal plants in the subtropical forests of Meghalaya, India. Rev Biol Trop 62:1295–1308. https://doi.org/10.15517/rbt.v62i4.12138
Weir T, Perry L, Gilroy S, Vivanco J (2010) The role of root exudates in rhizosphere interactions with plants and other organisms. Annu Rev Plant Biol. https://doi.org/10.1146/annurev.arplant.57.032905.105159
Zhao JL, Zhou LG, Wu JY (2010) Promotion of Salvia miltiorrhiza hairy root growth and tanshinone production by polysaccharide-protein fractions of plant growth-promoting rhizobacterium Bacillus cereus. Process Biochem 45:1517–1522. https://doi.org/10.1016/j.procbio.2010.05.034
Zubek S, Mielcarek S, Turnau K (2012) Hypericin and pseudohypericin concentrations of a valuable medicinal plant Hypericum perforatum L. are enhanced by arbuscular mycorrhizal fungi. Mycorrhiza 22:149–156. https://doi.org/10.1007/s00572-011-0391-1
Ali A, Mohanta TK, Asaf S, Rehman N, Al-Housni S, Al-Harrasi A, Khan AL, Al-Rawahi A (2019) Biotransformation of benzoin by Sphingomonas sp. LK11 and ameliorative effects on growth of Cucumis sativus. Arch Microbiol 201:591–601. https://doi.org/10.1007/s00203-019-01623-1
Author Information
Department of Genetic Engineering, SRM Institute of Science and Technology, Chennai, India