Root endophytic aquatic hyphomycetes from the Nandhaur River, India
Research Articles | Published: 21 October, 2024
First Page: 1249
Last Page: 1255
Views: 1719
Keywords: Aquatic hyphomycetes, Root endophytes, New hosts, New record, Nandhaur River
Abstract
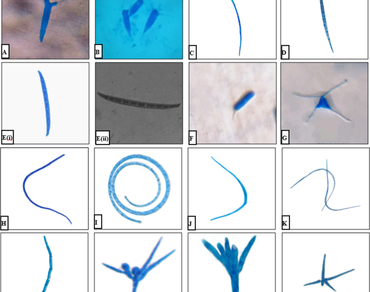
Nandhaur River, an unexplored water body in the Kumaun Himalayan foothill region, was investigated for the root endophytic aquatic hyphomycetes (REAHs) by the examination of collected root samples of healthy riparian plants through ‘Agar plating’ and ‘Direct detection’ methods. The study was carried out to explore the association of these fungi (REAHs) with different host plants and contribute to the diversity and distribution of these fungi in India. Altogether 15 species of aquatic hyphomycetes namely Acaulopage dichotoma, Acaulopage tetraceros, Amniculicola longissima, Anguillospora crassa, Anguillospora mediocris, Bacillispora aquatica, Campylospora filicladia, Flagellospora curvula, Helicomyces roseus, Lunulospora curvula, Setosynemma isthamosporum, Spieropsis scopiformis, Tetracladium marchalianum, Tetracladium setigerum and Trescelophorus acuminatus were isolated and identified as root endophytes of new host plants. A. mediocris was found to be a new addition to the Indian aquatic hyphomycetes, as it has not yet been reported from India. A. dichotoma, A. mediocris, C. filicladia, F. curvula and S. scopiformis were isolated as root endophytes for the first time from the foothill region of the Kumaun Himalaya. Maximum diversity was recorded in the autumn and winter seasons over the temperature range of 10–25 °C. The average incubation time for sporulation of these fungi was recorded as 10–35 days except for A. tetraceros, A. longissima, A. mediocris, B. aquatica and C. filicladia which sporulated in the latter stages of the incubation period. Mallotus philippensis was recorded as the best host plant as it favoured the growth of the maximum species of REAHs.
References
Altaf S, Bisht S, Jalal R, Kaur J (2022) Diversity and seasonal variation of aquatic hyphomycetes in Nandhaur wildlife sanctuary, Uttarakhand, India. Res J Agric Sci Int J 13:594–600
Barbosa FF, Gusmão LFP (2005) Two Speiropsis species (anamorphic fungi—hyphomycetes) from Bahia state, Brazil. Acta Bot Brasil 19:515–518. https://doi.org/10.1590/s0102-33062005000300012
Bärlocher F (2000) Water-borne conidia of aquatic hyphomycetes: seasonal and yearly patterns in Catamaran Brook, New Brunswick, Canada. Can J Bot 78:157–167. https://doi.org/10.1139/b99-172
Bills G, Polishook JD (1992) Recovery of endophytic fungi from Chamaecyparis thyoides. Sydowia 44:1–12
Chan S, Goh T, Hyde K (2000) Ingoldian fungi in Hong Kong. Fungal Divers 5:89–107
Fisher PJ, Anson A, Petrini O (1984) Antibiotic activity of some endophytic fungi from ericaceous plants. Bot Helv 94:249–253
Fisher PJ, Petrini O, Webster J (1991) Aquatic hyphomycetes and other fungi in living aquatic and terrestrial roots of Alnus glutinosa. Mycol Res 95:543–547. https://doi.org/10.1016/S0953-7562(09)80066-X
Gönczöl J, Marvanová L (2002) Anquillospora mediocris sp. Nov. from streams in Hungary. Czech Mycol 53:309–317. https://doi.org/10.3358/cmy.53407
Ingold CT (1942) Aquatic hyphomycetes of decaying alder leaves. Trans Br Mycol Soc 25:339-IN6. https://doi.org/10.1016/s0007-1536(42)80001-7
Ingold CT (1975) An illustrated guide to aquatic and water-borne hyphomycetes (fungi imperfecti) with notes on their biology. Freshw Biol Assoc Sci Publ 30:1–96. https://doi.org/10.2307/3758921
Kshirsagar AD, Gunale VR (2013) Diversity of aquatic fungi from Mula river at Pune city. Int J Adv Life Sci (Coimbatore) 6:174–184
Marvanova L, Fisher PJ (1991) A new endophytic hyphomycetes from alder roots. Nova Hedwigia 52:33–37
Mer GS, Sati SC (1989) Seasonal fluctuation in species composition of aquatic hyphomycetous flora in a temperate freshwater stream of central Himalaya, India. Int Rev Gesamten Hydrobiol Hydrogr 74:433–437. https://doi.org/10.1002/iroh.19890740405
Petrini O, Sieber TN, Toti L, Viret O (1993) Ecology, metabolite production, and substrate utilization in endophytic fungi. Nat Toxins 1:185–196. https://doi.org/10.1002/nt.2620010306
Saikawa M, Kadowaki T (2002) Studies on Acaulopage dichotoma and A. tetraceros (Zoopagales, Zygomycota) capturing amoebae. Nova Hedwigia 74:365–371. https://doi.org/10.1127/0029-5035/2002/0074-0365
Santos-Flores CJ, Betancourt-López C (1997) Aquatic and water-borne hyphomycetes (Deuteromycotina) in streams of Puerto Rico (including records from other neotropical locations). Caribb J Sci Spec pu 2:1–116
Sati S, Belwal M (2005) Aquatic hyphomycetes as endophytes of riparian plant roots. Mycologia 97:45–49. https://doi.org/10.1080/15572536.2006.11832837
Sati S, Bisht S (2006) Utilization of various carbon sources for the growth of waterborne conidial fungi. Mycologia 98:678–681. https://doi.org/10.3852/mycologia.98.5.678
Sati S, Pant P (2020) Two root endophytic aquatic hyphomycetes Campylospora parvula and Tetracladium setigerum as plant growth promoters. Asian J Agric Res 14:28–33. https://doi.org/10.3923/ajar.2020.28.33
Sati S, Pathak R (2017) New root endophytic water-borne conidial fungi from Kumaun Himalayas. Curr Bot 8:12–16
Sridhar KR, Bärlocher F (1992) Endophytic aquatic hyphomycetes of roots of spruce, birch and maple. Mycol Res 96:305–308. https://doi.org/10.1016/S0953-7562(09)80942-8
Sridhar KR, Bärlocher F (1993) Effect of temperature on growth and survival of five aquatic hyphomycetes. Sydowia 45:377–387
Waid J (1954) Occurrence of aquatic hyphomycetes upon the roots surface of beech grown in woodland soil. Trans Br Mycol Soc 37:420–421
Watanabe T (1975) Tetracladium stigerum an aquatic hyphomycetes associated with gentian and strawberry roots. Trans Mycol Soc Jpn 16:348–350
Author Information
Department of Botany, IPGG (PG) College of Commerce, Haldwani, Kumaun University, Nainital, India