Somatic embryogenesis induced from vascular tissues in leaf explants of Lisianthus (Eustoma grandiflorum (Raf.) Shinn) generates true-to-type diploid plants
Research Articles | Published: 03 January, 2020
First Page: 135
Last Page: 144
Views: 4001
Keywords: Eustoma sp., Flow cytometry, Gentianaceae, Ornamental plant, Procambium
Abstract
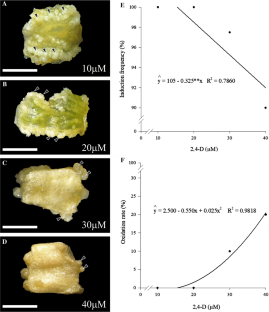
This study aimed to propose a somatic embryogenesis protocol for lisianthus (Eustoma grandiflorum) using leaf explants, as well as to characterize the cytological alterations involved in the regeneration process and ploidy fidelity of regenerated plants. The explants showed responsiveness and induced formation of pro-embryogenic structures in medium supplemented with 2,4-dichlorophenoxyacetic acid (2,4-D), reaching 100% of induction frequency at the concentrations of 10 and 20 µM. Somatic embryos differentiation occurred in both tested treatments: 6-benzyladenine (BA) and meta-Topolin (mT). The use of auxin and cytokinins on the somatic embryogenesis induction step generated true-to-type regenerants, maintaining the same ploidy levels. The plants obtained from both the differentiation of the somatic embryos and from seeds showed an average DNA amount of 3.53 pg, with and coefficient of variation of 2.9%, confirming that the regenerating plants are diploid (2n). Anatomical studies showed that the origin of somatic embryos is associated with the intense divisions of the vascular parenchyma. After transfer the somatic embryos to the differentiation and maturation media, there was an asynchronous germination and regeneration of in vitro lisianthus plants, with the best maturation occurred with 2 µM BA; 2 or 4 µM mT. This study opens new perspectives for somatic embryogenesis of lisianthus, so as to optimize the in vitro regeneration systems used in genetic breeding programs for this ornamental species.
References
- Amoo SO, Aremu AO, Moyo M, Szüčová L, Doležal K, Van Staden J (2013) Physiological effects of a novel aromatic cytokinin analogue in micropropagated Aloe arborescens and Harpagophytum procumbens. Plant Cell Tiss Organ Cult 116:17–26. https://doi.org/10.1007/s11240-013-0377-0
- Arnhold E (2014) Easyanova: analysis of variance and other important complementary analyzes. R package version 4.0. https://pt.scribd.com/document/376339016/easyanova. Accessed 15 Jan 2019
- Bairu MW, Aremu AO, van Staden J (2011) Somaclonal variation in plants: causes and detection methods. Plant Growth Regul 63:147–173. https://doi.org/10.1007/s10725-010-9554-x
- Bar OHL, Dawayati MM (2014) Histological changes on regeneration in vitro culture of date palm (Phoenix dactylifera) leaf explants. Aust J Crop Sci 8:848–855
- Barešová H, Kaminek M (1984) Light induced embryogenesis in suspension culture of Centaurium erythraea Rafn. In: Novak FJ, Havel JL, Doležel J (eds) Plant tissue and cell culture propagation to crop improvement. Czech Academy of Sciences, Prague, pp 163–164
- Barrueto Cid LP, Teixeira J (2006) Indução de organogênese em lisianthus, Eustoma grandiflorum, a partir de fragmentos foliares cultivados in vitro. Boletim de Pesquisa e Desenvolvimento/Embrapa Recursos Genéticos e Biotecnologia, Brasilia
- Caboni E, Tonelli M, Lauri P, D’Angeli S, Damiano C (1999) In vitro shoot regeneration from leaves of wild pear. Plant Cell Tissue Org Cult 59:1–7. https://doi.org/10.1023/A:1006351214343
- Cipriano JLD, Cruz ACF, Mancini KC, Schmildt ER, Lopes JC, Otoni WC, Alexandre RS (2018) Somatic embryogenesis in Carica papaya as affected by auxins and explants, and morphoanatomical-related aspects. An Acad Bras Cienc 90:385–400. https://doi.org/10.1590/0001-3765201820160252
- Deroles SC, Ledger SE, Miller RM, Davies KM, Given NK (1993) Transformation in Eustoma grandiflorum (lisianthus). In: Bajaj YPS (eds) Plant protoplasts and genetic engineering III. Biotechnology in Agriculture and Forestry, Vol 22. Springer, Berlin. doi: https://doi.org/10.1007/978-3-642-78006-6_18
- Dole JM, Wilkins HF (2005) Eustoma. Floriculture: principles and species. Pearson Prentice Hall, New Jersey, pp 514–521
- Doležel J, Bartos J (2005) Plant DNA flow cytometry and estimation of nuclear genome size. Ann Bot 95:99–110. https://doi.org/10.1093/aob/mci005
- Doležel J, Greilhuber J, Lucretti S, Meister A, Lysa´k MA, Nardi L, Obermayer R (1998) Plant genome size estimation by flow cytometry: inter-laboratory comparison. Ann Bot 82(Suppl. A):17–26. https://doi.org/10.1093/oxfordjournals.aob.a010312
- Elmeer SKE (2013) Factors regulating somatic embryogenesis in plants. In: Aslam J, Srivastava PS, Sharma MP (eds) Somatic embryogenesis and gene expression, 1st edn. Narosa, New Delhi, pp 56–81
- Esizad SG, Kaviani B, Alireza T, Sahar BZ (2012) Micropropagation of Lisianthus (Eustoma grandiflorum) an ornamental plant. Plant Omics J 5:314–319
- Fehér A, Pasternak TP, Dudits D (2003) Transition of somatic plant cells to an embryogenic state. Plant Cell Tissue Org Cult 74:201–228. https://doi.org/10.1023/A:1024033216561
- Ferreira EB, Cavalcanti PP, Nogueira DA (2013) ExpDes: Experimental Designs package. Version 1.1.2
- Filipovic B, Simonovic A, Trifunovic M, Dmitrovic S, Savic J, Jevremovic S, Subotic A (2015) Plant regeneration in leaf culture of Centaurium erythraea Rafn. Part 1: the role of antioxidant enzymes. Plant Cell Tissue Org Cult 121:703–719
- Fiuk A, Rybczyński JJ (2008) Genotype and plant growth regulator-dependent response of somatic embryogenesis from Gentiana spp. leaf explants. In Vitro Cell Dev Biol Plant 44:90–99. https://doi.org/10.1007/s11627-008-9124-3
- Fukuda H (2004) Signals that control plant vascular cell differentiation. Nat Rev Mol Cell Biol 5:379–391. https://doi.org/10.1038/nrm1364
- Gaj MD (2004) Factors influencing somatic embryogenesis induction and plant regeneration with particular reference to Arabidopsis thaliana (L.) Heynh. Plant Growth Regul 43:27–47. https://doi.org/10.1023/B:GROW.0000038275.29262.fb
- Gentile A, Jàquez Gutiérrez M, Martinez J, Frattarelli A, Nota P, Caboni E (2014) Effect of meta-Topolin on micropropagation and adventitious shoot regeneration in Prunus rootstocks. Plant Cell Tissue Org Cult 118:373–381. https://doi.org/10.1007/s11240-014-0489-1
- Gesteira AS, Otoni WC, Barros EG, Moreira MA (2002) RAPD- based detection of genomic instability in soybean plants derived from somatic embryogenesis. Plant Breed 121:269–271. https://doi.org/10.1046/j.1439-0523.2002.00708.x
- Haque SM, Ghosh B (2014) Somatic embryogenesis and synthetic seed production—a biotechnological approach for true-to-type propagation and in vitro conservation of an ornamental bulbaceous plant Drimiopsis kirkii Baker. Appl Biochem Biotechnol 172:4013–4024. https://doi.org/10.1007/s12010-014-0817-2
- Handa T, Deroles SC (2001) Transgenic Eustoma grandiflorum (Lisianthus). In: Bajaj YPS (ed) Transgenic crops III. Springer, Berlin, pp 107–122
- Harbaugh BK (2007) Lisianthus Eustoma grandiflorum. In: Anderson NO (ed) Flower breeding and genetics: issues, challenges and opportunities for the 21st century. Springer, Amsterdam, pp 644–663
- Harbaugh BK, Scott JW (2003) “Maurine Twilight” and “Maurine Daylight”–heat-tolerant lisianthus with bi-colored flowers. HortScience 31:131–132
- He T, Yang L, Zhao Z (2011) Embryogenesis of Gentiana straminea and assessment of genetic stability of regenerated plants using inter simple sequence repeat (ISSR) marker. Afr J Biotechnol 10:7604–7610. https://doi.org/10.5897/AJB11.572
- Ikeuchi M, Ogawa Y, Iwase A, Sugimoto K (2016) Plant regeneration: cellular origins and molecular mechanisms. Development 143:1442–1451. https://doi.org/10.1242/dev.134668
- Jha BT, Dafadar A, Chaudhur RK (2011) Somatic embryogenesis in Swertia chirata Buch. Ham. Ex Wall.- a multipotent medicinal plant. Asian J Biotechnol 3:186–193. https://doi.org/10.3923/ajbkr.2011.186.19.3
- Junqueira AH, Peetz MS (2017) Brazilian consumption of flowers and ornamental plants: habits, practices and trends. Ornam Hortic 23:178–184. https://doi.org/10.14295/oh.v23i2.1070
- Karnovsky MJ (1965) A formaldehyde-glutaraldehyde fixative of high osmolality for use in electron microscopy. J Cell Biol 27:137–138
- Kaviani B (2014) Micropropagation of ten weeks (Matthiola incana) and lisianthus (Eustoma grandiflorum) (two ornamental plants) by using kinetin (Kin), naphthalene acetic acid (NAA) and 2,4-dichlorophenoxyacetic acid (2,4-D). Acta Sci Pol-Hortoru 13:141–154
- Lai S, Menon A, Turner S, Kodym A, Bunn E (2013) Development of an in vitro protocol for a difficult-to-propagate endemic Australian dryland sedge species Mesomelaena pseudostygia (Cyperaceae). In Vitro Cell Dev Biol Plant 50:99–109. https://doi.org/10.1007/s11627-013-9542-8
- Loureiro J, Rodriguez E, Doležel J, Santos C (2007) Two new nuclear isolation buffers for plant DNA flow cytometry: a test with 37 species. Ann Bot 100:875–888. https://doi.org/10.1093/aob/mcm152
- Lugassi-Ben-Hamo M, Kitron M, Bustan A, Zaccai M (2010) Effect of shade regime on flower development, yield and quality in lisianthus. Sci Hortic 124:248–253. https://doi.org/10.1016/j.scienta.2009.12.030
- Mikuła A, Rybczyński JJ (2001) Somatic embryogenesis of Gentiana genus I. The effect of the preculture treatment and primary explant origin on somatic embryogenesis of Gentiana cruciata (L.), G. pannonica Acta Physiol Plant 23:15–25. https://doi.org/10.1007/s11738-001-0017-x
- Murashige T, Skoog F (1962) A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plant 15:473–497
- Nhut DT, Tuan NS, Ngoc HM, Uyen PN, Don NT, Mai NT, Teixeira da Silva J (2006) Somatic embryogenesis induction from in vitro leaf culture of lisianthus (Eustoma grandiflorum (Raf.) Shinn.). Prop Ornam Plants 6:121–127
- O’Brien TP, McCully ME (1981) The study of plant structure principles and selected methods. Termarcarphi Pty Ltd., Melbourne
- Ojtania AGW (2010) Effect of meta-topolin on in vitro propagation of Pelargonium × Hortorum and Pelargonium × Hederaefolium cultivars. Acta Soc Bot Pol 79:101–106. https://doi.org/10.5586/asbp.2010.013
- Ördögh M‚ Jambor-Benczur E, Tilly-Mandy A (2006) Micropropagation of Echo cultivars of Eustoma grandiflorum. Acta Hortic 725:457–460. https://doi.org/10.17660/ActaHortic.2006.725.64
- Paek KY, Hahn EJ (2000) Cytokinins, auxins and activated charcoal affect organogenesis and anatomical characteristics of shoot-tip cultures of lisianthus [Eustoma grandiflorum (Raf.) Shinn]. In Vitro Cell Dev Biol Plant 36:128–132. https://doi.org/10.1007/s11627-000-0026-2
- R Core Team (2014) Software R: a language and environment for statistical computing version 3.0.3. https://repo.bppt.go.id/cran/web/packages/dplR/vignettes/intro-dplR.pdf. Accessed 21 Jan 2019
- Rabobank (2015) Floriculture Map 2015. Rabobank Industry Note 475. http://www.florisud.fr/var/florisud/storage/original/application/21f23b81f7f4301304ffd6e2485ba7a6.pdf. Accessed 4 Feb 2019
- Rabobank (2016) World Floriculture Map 2016: equator countries gathering speed. https://research.rabobank.com/far/en/sectors/regional-food-agri/world_floriculture_map_2016.html. Acessed 27 July 2018.
- Rakoczy-Trojanowska M (2002) The effects of growth regulators on somaclonal variation in rye (Secale cereale L.) and selection of somaclonal variants with increased agronomic traits. Cell Mol Biol Lett 7:1111–1120
- Rathore MS, Paliwal N, Anand KGV, Agarwal PK (2015) Somatic embryogenesis and in vitro plantlet regeneration in Salicornia brachiata Roxb. Plant Cell Tissue Org Cult 120:355–360. https://doi.org/10.1007/s11240-014-0571-8
- Rezaee F, Faezeh G, Laleh YB (2012) Micropropagation of Lisianthus (Eustoma grandiflorum L.) from different explants to flowering onset. Iran J Plant Physiol 3:583–587
- Rocha DI, Vieira LM, Koehler AD, Otoni WC (2018) Cellular and morpho-histological foundations of in vitro plant regeneration. In: Loyola-Vargas VM, Ochoa-Alejo N (eds) Plant cell culture protocols, methods in molecular biology, pp 47–68. doi: https://doi.org/10.1007/978-1-4939-8594-4_3
- Rosa YBCJ, Monte-Bello CC, Dornelas MC (2015) Species-dependent divergent responses to in vitro somatic embryo induction in Passiflora spp. Plant Cell Tissue Org Cult 120:69–77. https://doi.org/10.1007/s11240-014-0580-7
- Ruffoni B, Bassolino L (2016) Somatic embryogenesis in Lisianthus (Eustoma russellianum Griseb.). In: Germanà MA, Lambardi M (eds) In vitro embryogenesis in higher plants. Methods in molecular biology. Humana Press, New York, pp 359–370
- Ruffoni B, Damiano C, Massabò F, Esposito P (1990) Organogenesis and embryogenesis in Lisianthus russellianus Hook. Acta Hortic 280:83–88. https://doi.org/10.17660/ActaHortic.1990.280.11
- Semeria L, Ruffoni B, Rabaglio M, Genga A, Vaira AM, Accotto GP, Allavena A (1996) Genetic transformation of Eustoma grandiflorum by Agrobacterium tumefaciens Plant Cell Tissue Org Cult 47:67–72. https://doi.org/10.1007/BF02318967
- Sharma SK, Millam S (2004) Somatic embryogenesis in Solanum tuberosum L.: a histological examination of key developmental stages. Plant Cell Rep 23:115–119. https://doi.org/10.1007/s00299-004-0814-y
- Shibli RA, Duwayri MA, Sawwan JS, Shatnawi MA, Al-Qudah TS (2012) Regeneration via somatic embryogenesis of the endangered wild arum (Arum palaestinum). In Vitro Cell Dev Biol Plant 48:335–340. https://doi.org/10.1007/s11627-012-9438-z
- Sivanesan I, Lim MY, Jeong BR (2011) Somatic embryogenesis and plant regeneration from leaf and petiole explants of Campanula punctata Lam. var. rubriflora Makino. Plant Cell Tissue Org Cult 107:365–369. https://doi.org/10.1007/s11240-011-9983-x
- Tomiczak K, Mikuła A, Niedziela A, Wójcik-Lewandowska A, Domzalska L, Rybczynski JJ (2019) Somatic embryogenesis in the family gentianaceae and its biotechnological application. Front Plant Sci 10:762. https://doi.org/10.3389/fpls.2019.00762
- Valero-Aracama C, Kane ME, Wilson SB, Philman NL (2009) Substitution of benzyladenine with meta-topolin during shoot multiplication increases acclimatization of difficult- and easy-to-acclimatize sea oats (Uniola paniculata L.) genotypes. Plant Growth Regul 60:43–49. https://doi.org/10.1007/s10725-009-9417-5
- Von Arnold S (2008) Somatic embryogenesis. In: George EF, Hall MA, De Klerk G-J (eds) Plant propagatation by tissue culture, 3rd edn. Springer, Amsterdam, pp 335–354
- Von Arnold S, Sabala I, Bozhkov P, Dyachok J, Filonova L (2002) Developmental pathways of somatic embryogenesis. Plan Cell Tissue Org Cult 69:233–249. https://doi.org/10.1023/A:1015673200621
- Wang XD, Nolan KE, Irwanto RR, Sheahan MB, Rose RJ (2011) Ontogeny of embryogenic callus in Medicago truncatula: the fate of the pluripotent and totipotent stem cells. Ann Bot 107:599–607. https://doi.org/10.1093/aob/mcq269
- Wang H, Li M, Yang Y, Dong J, Jin W (2015) Histological and endogenous plant growth regulators changes associated with adventitious shoot regeneration from in vitro leaf explants of strawberry (Fragaria x ananassa cv. ‘Honeoye’). Plant Cell Tissue Org Cult 123:479–488. https://doi.org/10.1007/s11240-015-0851-y
- Weckx S, Inzé D, Maene L (2019) Tissue culture of oil palm: finding the balance between mass propagation and somaclonal variation. Front Plant Sci 10:722. https://doi.org/10.3389/fpls.2019.00722
Author Information
Departamento de Fitotecnia, Universidade Federal de Viçosa, Viçosa, Brazil