Study of biochemical and biophysical adjustments during transition from desiccation-to-fully-hydrated states in Riccia gangetica and Semibarbula orientalis
Research Articles | Published: 27 June, 2022
First Page: 550
Last Page: 558
Views: 3593
Keywords: Abiotic stress, Desiccation, Bryophytes, Semibarbula orientalis , Riccia gangetica , Antioxidants, Photosynthesis, Chlorophyll a fluorescence, Reactive oxygen species, Hypo-osmotic shock
Abstract
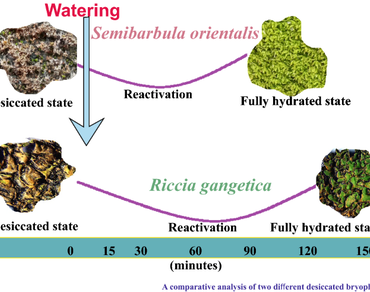
References
Anwar Hossain M, Hoque MA, Burritt DJ, Fujita M (2014) Proline protects plants against abiotic oxidative stress: biochemical and molecular mechanisms. In: oxidative damage to plants: antioxidant networks and signaling
Apel K, Hirt H (2004) Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu Rev Plant Biol 55:373–399
Arnon DI (1949) Copper enzymes in isolated chloroplasts. Polyphenoloxidase in beta vulgaris. Plant Physiol. https://doi.org/10.1104/pp.24.1.1
Asami P, Mundree S, Williams B (2018) Saving for a rainy day: control of energy needs in resurrection plants. Plant Sci 271:62–66
Ashraf M, Foolad MR (2007) Roles of glycine betaine and proline in improving plant abiotic stress resistance. Environ Exp Bot. https://doi.org/10.1016/j.envexpbot.2005.12.006
Augusti A, Scartazza A, Navari-Izzo F et al (2001) Photosystem II photochemical efficiency, zeaxanthin and antioxidant contents in the poikilohydric Ramonda serbica during dehydration and rehydration. Photosynth Res. https://doi.org/10.1023/A:1010692632408
Bates LS, Waldren RP, Teare ID (1973) Rapid determination of free proline for water-stress studies. Plant Soil. https://doi.org/10.1007/BF00018060
Bewley JD (1979) Physiological aspects of desiccation tolerance. Annu Rev Plant Physiol. https://doi.org/10.1146/annurev.pp.30.060179.001211
Bhatt U, Singh H, Kumar D, Soni V (2019) Rehydration induces quick recovery of photosynthesis in desiccation tolerant moss Semibarbula orientalis. J Plant Sci Res 35
Blokhina O, Virolainen E, Fagerstedt KV (2003) Antioxidants, oxidative damage and oxygen deprivation stress: a review. Ann Bot 91:179–194
Brown RC, Lemmon BE (2011) Dividing without centrioles: Innovative plant microtubule organizing centres organize mitotic spindles in bryophytes, the earliest extant lineages of land plants. AoB Plants. https://doi.org/10.1093/aobpla/plr028
Challabathula D, Puthur JT, Bartels D (2016) Surviving metabolic arrest: photosynthesis during desiccation and rehydration in resurrection plants. Ann N Y Acad Sci. https://doi.org/10.1111/nyas.12884
Chang Y, Graham SW (2011) Inferring the higher-order phylogeny of mosses (Bryophyta) and relatives using a large, multigene plastid data set. Am J Bot. https://doi.org/10.3732/ajb.0900384
Crandall-Stotler B, Stotler RE (2012) Morphology and classification of the Marchantiophyta. In: Bryophyte biology
Cruz de Carvalho R, Catalá M, Branquinho C et al (2017) Dehydration rate determines the degree of membrane damage and desiccation tolerance in bryophytes. Physiol Plant. https://doi.org/10.1111/ppl.12511
Csintalan Z, Tuba Z, Lichtenthaler HK, Grace J (1996) Reconstitution of photosynthesis upon rehydration in the desiccated leaves of the Poikilochlorophyllous shrub Xerophyta scabrida at elevated CO2. J Plant Physiol. https://doi.org/10.1016/S0176-1617(96)80263-X
de León IP, Montesano M (2013) Activation of defense mechanisms against pathogens in mosses and flowering plants. Int J Mol Sci 14:3178–3200
De Ronde JA, Cress WA, Krüger GHJ et al (2004) Photosynthetic response of transgenic soybean plants, containing an Arabidopsis P5CR gene, during heat and drought stress. J Plant Physiol. https://doi.org/10.1016/j.jplph.2004.01.014
Dey A, De JN (2012) Antioxidative potential of bryophytes: stress tolerance and commercial perspectives: a review. Pharmacologia. https://doi.org/10.5567/pharmacologia.2012.151.159
Duckett JG, Ligrone R (1995) The formation of catenate foliar gemmae and the origin of oil bodies in the liverwort odontoschisma denudatum (Mart.) dum. (Jungermanniales): a light and electron microscope study. Ann Bot. https://doi.org/10.1006/anbo.1995.1114
Efeoǧlu B, Ekmekçi Y, Çiçek N (2009) Physiological responses of three maize cultivars to drought stress and recovery. S Afr J Bot. https://doi.org/10.1016/j.sajb.2008.06.005
Farrant JM, Cooper K, Hilgart A et al (2015) A molecular physiological review of vegetative desiccation tolerance in the resurrection plant Xerophyta viscosa (Baker). Planta 242:407–426
Flores JR, Catalano SA, Muñoz J, Suárez GM (2018) Combined phylogenetic analysis of the subclass Marchantiidae (Marchantiophyta): towards a robustly diagnosed classification. Cladistics. https://doi.org/10.1111/cla.12225
Flores JR, Suárez GM, Hyvönen J (2020) Reassessing the role of morphology in bryophyte phylogenetics: combined data improves phylogenetic inference despite character conflict. Mol Phylogenet Evol. https://doi.org/10.1016/j.ympev.2019.106662
Forni C, Duca D, Glick BR (2017) Mechanisms of plant response to salt and drought stress and their alteration by rhizobacteria. Plant Soil. https://doi.org/10.1007/s11104-016-3007-x
Fryer MJ, Ball L, Oxborough K et al (2003) Control of ascorbate peroxidase 2 expression by hydrogen peroxide and leaf water status during excess light stress reveals a functional organisation of Arabidopsis leaves. Plant J. https://doi.org/10.1046/j.1365-313X.2003.01656.x
Ghosh UK, Islam MN, Siddiqui MN et al (2022) Proline, a multifaceted signalling molecule in plant responses to abiotic stress: understanding the physiological mechanisms. Plant Biol 24:227–239
Gururani MA, Venkatesh J, Ganesan M et al (2015) In Vivo assessment of cold tolerance through chlorophyll-a fluorescence in transgenic zoysiagrass expressing mutant phytochrome A. PLoS One 10(5):e0127200. https://doi.org/10.1371/journal.pone.0127200
Harris BJ, Harrison CJ, Hetherington AM, Williams TA (2020) Phylogenomic evidence for the monophyly of bryophytes and the reductive evolution of stomata. Curr Biol. https://doi.org/10.1016/j.cub.2020.03.048
Heber U, Soni V, Strasser RJ (2011) Photoprotection of reaction centers: thermal dissipation of absorbed light energy vs charge separation in lichens. Physiol Plant. https://doi.org/10.1111/j.1399-3054.2010.01417.x
Jovanović Ž, Rakić T, Stevanović B, Radović S (2011) Characterization of oxidative and antioxidative events during dehydration and rehydration of resurrection plant Ramonda nathaliae. Plant Growth Regul. https://doi.org/10.1007/s10725-011-9563-4
Kaiser WM (1979) Reversible inhibition of the calvin cycle and activation of oxidative pentose phosphate cycle in isolated intact chloroplasts by hydrogen peroxide. Planta. https://doi.org/10.1007/BF00388364
Kalaji HM, Schansker G, Ladle RJ et al (2014) Frequently asked questions about in vivo chlorophyll fluorescence: practical issues. Photosynth Res 122:121–158
Kono Y (1978) Generation of superoxide radical during autoxidation of hydroxylamine and an assay for superoxide dismutase. Arch Biochem Biophys. https://doi.org/10.1016/0003-9861(78)90479-4
Ligrone R, Duckett JG, Renzaglia KS (2012) Major transitions in the evolution of early land plants: a bryological perspective. Ann Bot 109:851–871
Mansoor S, Ali Wani O, Lone JK et al (2022) Reactive oxygen species in plants: from source to sink. Antioxidants 11:225
Mattson WJ, Haack RA (1987) The role of drought in outbreaks of plant-eating insects. Bioscience. https://doi.org/10.2307/1310365
Merced A, Renzaglia KS (2017) Structure, function and evolution of stomata from a bryological perspective. Bryophyt Divers Evol 39:7–20
Mihailova G, Vasileva I, Gigova L et al (2022) Antioxidant defense during recovery of resurrection plant Haberlea rhodopensis from drought-and freezing-induced desiccation. Plants 11:175
Mundree SG, Whittaker A, Thomson JA, Farrant JM (2000) An aldose reductase homolog from the resurrection plant Xerophyta viscosa baker. Planta. https://doi.org/10.1007/s004250000331
Oliver MJ, Dowd SE, Zaragoza J et al (2004) The rehydration transcriptome of the desiccation-tolerant bryophyte Tortula ruralis: transcript classification and analysis. BMC Genom. https://doi.org/10.1186/1471-2164-5-89
Oliver MJ, Derek Bewley J (2010) Desiccation-tolerance of plant tissues: a mechanistic overview. In: Horticultural reviews
Onele AO, Chasov A, Viktorova L et al (2018) Biochemical characterization of peroxidases from the moss Dicranum scoparium. S Afr J Bot. https://doi.org/10.1016/j.sajb.2018.08.014
Parvaiz A, Satyawati S (2008) Salt stress and phyto-biochemical responses of plants—a review. Plant Soil Environ 54:89
Proctor MCF, Oliver MJ, Wood AJ et al (2007) Desiccation-tolerance in bryophytes: a review. Bryologist 110:595–621
Qiu YL (2008) Phytogeny and evolution of charophytic algae and land plants. J Syst Evol. https://doi.org/10.3724/SP.J.1002.2008.08035
Qiu YL, Li B, Wang B et al (2007) A nonflowering land plant phylogeny inferred from nucleotide sequences of seven chloroplast, mitochondrial, and nuclear genes. Int J Plant Sci. https://doi.org/10.1086/513474
Racusen D, Foote M (1965) Protein synthesis in dark-grown bean leaves. Can J Bot. https://doi.org/10.1139/b65-091
Rakić T, Lazarević M, Jovanović ŽS et al (2014) Resurrection plants of the genus Ramonda: prospective survival strategies—unlock further capacity of adaptation, or embark on the path of evolution? Front Plant Sci 4:550
Sánchez-Rodríguez E, Mm R-W, Cervilla LM et al (2010) Genotypic differences in some physiological parameters symptomatic for oxidative stress under moderate drought in tomato plants. Plant Sci. https://doi.org/10.1016/j.plantsci.2009.10.001
Scandalios JG (1993) Oxygen stress and superoxide dismutases. Plant Physiol 101:7
Schlensog M, Schroeter B (2001) A new method for the accurate in situ monitoring of chlorophyll a fluorescence in lichens and bryophytes. Lichenol 33:443–452
Sgherri C, Stevanovic B, Navari-Izzo F (2004) Role of phenolics in the antioxidative status of the resurrection plant Ramonda serbica during dehydration and rehydration. Physiol Plant. https://doi.org/10.1111/j.1399-3054.2004.00428.x
Soni V (2020) Rehydration quickly assembles photosynthetic complexes in desiccation tolerant Riccia gangetica. Biomed J Sci Tech Res. https://doi.org/10.26717/bjstr.2020.30.004891
Strasser BJ, Strasser RJ (1995) Measuring fast fluorescence transients to address environmental questions: the JIP-test. In: Photosynthesis: from light to biosphere
Szabados L, Savouré A (2010) Proline: a multifunctional amino acid. Trends Plant Sci 15:89–97
Teranishi Y, Tanaka A, Osumi M, Fukui S (1974) Catalase activities of hydrocarbon-utilizing candida yeasts. Agric Biol Chem. https://doi.org/10.1080/00021369.1974.10861301
Todaka D, Shinozaki K, Yamaguchi-Shinozaki K (2015) Recent advances in the dissection of drought-stress regulatory networks and strategies for development of drought-tolerant transgenic rice plants. Front Plant Sci 6:84
Tsimilli-Michael M, Strasser RJ (2001) Fingerprints of climate changes on the photosynthetic apparatus’ behaviour, monitored by the JIP-test. In: “Fingerprints” of climate change
Turner NC (1981) Techniques and experimental approaches for the measurement of plant water status. Plant Soil. https://doi.org/10.1007/BF02180062
Urano K, Maruyama K, Jikumaru Y et al (2017) Analysis of plant hormone profiles in response to moderate dehydration stress. Plant J. https://doi.org/10.1111/tpj.13460
van der Walt K, Burritt DJ, Nadarajan J (2022) Impacts of rapid desiccation on oxidative status, ultrastructure and physiological functions of Syzygium maire (Myrtaceae) zygotic embryos in preparation for cryopreservation. Plants 11:1056
Velikova V, Yordanov I, Edreva A (2000) Oxidative stress and some antioxidant systems in acid rain-treated bean plants protective role of exogenous polyamines. Plant Sci. https://doi.org/10.1016/S0168-9452(99)00197-1
Veljovic-Jovanovic S, Kukavica B, Stevanovic B, Navari-Izzo F (2006) Senescence- and drought-related changes in peroxidase and superoxide dismutase isoforms in leaves of Ramonda serbica. J Exp Bot 57:1759–1768
Verslues PE, Agarwal M, Katiyar-Agarwal S et al (2006) Methods and concepts in quantifying resistance to drought, salt and freezing, abiotic stresses that affect plant water status. Plant J. https://doi.org/10.1111/j.1365-313X.2005.02593.x
Verslues PE, Lasky JR, Juenger TE et al (2014) Genome-wide association mapping combined with reverse genetics identifies new effectors of low water potential-induced proline accumulation in Arabidopsis. Plant Physiol. https://doi.org/10.1104/pp.113.224014
Wang X, Chen S, Zhang H et al (2010) Desiccation tolerance mechanism in resurrection fern-ally Selaginella tamariscina revealed by physiological and proteomic analysis. J Proteome Res. https://doi.org/10.1021/pr100767k
Wang H, Tang X, Wang H, Shao HB (2015) Proline accumulation and metabolism-related genes expression profiles in Kosteletzkya virginica seedlings under salt stress. Front Plant Sci. https://doi.org/10.3389/fpls.2015.00792
Wanjiku JG, Bohne H (2017) Growth and drought responses of three Prunus spinosa L. ecotypes. Afr J Hort Sci 12:40–50
You J, Chan Z (2015) Ros regulation during abiotic stress responses in crop plants. Front Plant Sci 6:1092
Zhanassova K, Kurmanbayeva A, Gadilgereyeva B et al (2021) ROS status and antioxidant enzyme activities in response to combined temperature and drought stresses in barley. Acta Physiol Plant. https://doi.org/10.1007/s11738-021-03281-7
Zhou X, Zheng Y, Wang L et al (2022) SYP72 interacts with the mechanosensitive channel MSL8 to protect pollen from hypoosmotic shock during hydration. Nat Commun 13:1–14
Author Information
Plant Bioenergetics and Biotechnology Laboratory, Department of Botany, Mohanlal Sukhadia University, Udaipur, India