Study of DWARF sequence of different plants to understand the probable host plants for mycorrhizal associations
*Article not assigned to an issue yet
Research Articles | Published: 22 July, 2025
First Page: 0
Last Page: 0
Views: 3
Keywords: Association, Hosts mycorrhizal association, Strigolactone receptor, DWARF sequence
Abstract
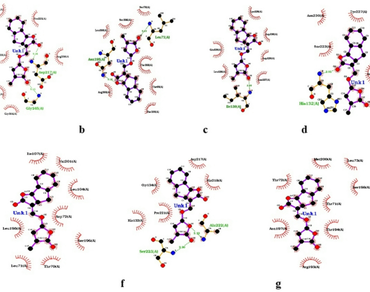
The mycorrhizal association is the plant-fungal association. This association is important to absorb water and essential minerals from the soil. Mycorrhiza production technology involves finding a suitable host for the fungal species. This process needs a lot of time and skill. A bioinformatics study has been done to find a suitable host from a wild plant’s mycorrhizal fungal partner. Strigolactone is the key component involved in the host and fungal partner compatibility. The strigolactone receptor (DWARF 14; Accession No: QMX78397.1) sequence was retrieved from the database and the homologs using BLAST. The phylogenetic analysis of the sequences was done to see the relatedness. An assessment was also done to find the affinity of strigolactone with receptors. Phylogenetic analysis shows that plants can be grouped according to the family, genus, and types of cotyledon. The affinity assay result predicts that positive binding affinity for all the DWARF 14 sequences of different plants to the strigolactones receptor. The affinities of DWARF sequences range from − 5.8 to -6.6 and thus indicate the stronger affinity of binding possibility to the ligand-receptor molecules. The process of finding before the experiment could be a novel approach towards initial host plant identification in less time and precise manner.
References
Al-Babili S, Bouwmeester HJ (2015) Strigolactones, a novel carotenoid-derived plant hormone. Annu Rev Plant Biol 66:161–186. https://doi.org/10.1146/annurev-arplant-043014-114759
Andrade SAL, Malik S, Sawaya ACHF, Bottcher A, Mazzafera P (2013) Association with arbuscular mycorrhizal fungi influences alkaloid synthesis and accumulation in Catharanthus roseus and Nicotiana tabacum plants. Acta Physiol Plant 35:867–880. https://doi.org/10.1007/s11738-012-1130-8
Balzergue C, Chabaud M, Barker DG, Bécard G, Rochange SF (2013) High phosphate reduces host ability to develop arbuscular mycorrhizal symbiosis without affecting root calcium spiking responses to the fungus. Front Plant Sci 4:426. https://doi.org/10.3389/fpls.2013.00426
Borghi L, Liu GW, Emonet A, Kretzschmar T, Martinoia E (2016) The importance of Strigolactone transport regulation for symbiotic signaling and shoot branching. Planta 243:1351–1360. https://doi.org/10.1007/s00425-016-2503-9
Brandsdal BO, Österberg F, Almlöf M, Feierberg I, Luzhkov VB, Åqvist J (2003) Free energy calculations and ligand binding. Advances in protein chemistry. 66(03):123–158. https://doi.org/10.1016/S0065-3233
Brun G, Braem L, Thoiron S, Gevaert K, Goormachtig S, Delavault P (2018) Seed germination in parasitic plants: what insights can we expect from Strigolactone research? J Exp Bot 69(9):2265–2280. https://doi.org/10.1093/jxb/erx472
Chen C, Zou J, Zhang S, Zaitlin D, Zhu L (2009) Strigolactones are a new-defined class of plant hormones which inhibit shoot branching and mediate the interaction of plant-AM fungi and plant-parasitic weeds. Sci China Ser C: Life Sci 52:693–700. https://doi.org/10.1007/s11427-009-0104-6
Chow B, McCourt P (2006) Plant hormone receptors: perception is everything. Genes Dev 20(15):1998–2008
Das D, Dutta G, Jahnavi J, Patra P, Bhuniya O, Ramlal A, Samanta A (2024) In Silico study of some bioactive compounds of Pleurotus sajor-caju as antidiabetic and antiviral agents. Nutrire 49(1):17. https://doi.org/10.1186/s41110-024-00256-9
De Jong LA, Uges DR, Franke JP, Bischoff R (2005) Receptor–ligand binding assays: technologies and applications. J Chromatogr B 829(1–2):1–25. https://doi.org/10.1016/j.jchromb.2005.10.002
Garrido E, Bennett AE, Fornoni J, Strauss SY (2010) The dark side of the mycorrhiza. Plant Signal Behav 5(8):1019–1021. https://doi.org/10.4161/psb.5.8.12292
Gascuel O (1997) BIONJ: an improved version of the NJ algorithm based on a simple model of sequence data. Mol Biol Evol 14(7):685–695. https://doi.org/10.1093/oxfordjournals.molbev.a025808
Giovannetti M, Volpe V, Salvioli A, Bonfante P (2017) Fungal and plant tools for the uptake of nutrients in arbuscular mycorrhizas: a molecular view. In: Johnson, N.C., Gehring, C., & Jansa, J. (eds) Mycorrhizal mediation of soil. pp. 107–128, Elsevier. https://doi.org/10.1016/B978-0-12-804312-7.00007-3
Guedes IA, de Magalhães CS, Dardenne LE (2014) Receptor–ligand molecular Docking. Biophys Rev 6:75–87. https://doi.org/10.1007/s12551-013-0130-2
Guindon S, Dufayard JF, Lefort V, Anisimova M, Hordijk W, Gascuel O (2010) New algorithms and methods to estimate maximum-likelihood phylogenies: assessing the performance of PhyML 3.0. Syst Biol 59(3):307–321. https://doi.org/10.1093/sysbio/syq010
Hashem HA, Khalil R (2023) Insight into the interaction of strigolactones, abscisic acid, and reactive oxygen species signals. In: Faizan M, Hayat S, Ahmad SM (eds) Reactive oxygen species: prospects in plant metabolism. Springer Nature Singapore, Singapore, pp 179–211
Huerta-Cepas J, Serra F, Bork P (2016) ETE 3: reconstruction, analysis, and visualization of phylogenomic data. Mol Biol Evol 33(6):1635–1638. https://doi.org/10.1093/molbev/msw046
Kapulnik Y, Koltai H (2014) Strigolactone involvement in root development, response to abiotic stress, and interactions with the biotic soil environment. Plant Physiol 166(2):560–569. https://doi.org/10.1104/pp.114.244939
Kollur SP, Prasad SK, Pradeep S, Veerapur R, Patil SS, Amachawadi RG, Prasad SR, Lamraoui G, Al-Kheraif AA, Elgorban AM, Syed A, Shivamallu C (2021) Luteolin-fabricated ZnO nanostructures showed PLK-1 mediated anti-breast cancer activity. Biomolecules 11(3):385. https://doi.org/10.3390/biom11030385
Laskowski RA, Swindells MB (2011) LigPlot+: multiple ligand–protein interaction diagrams for drug discovery. J ChemInf Model 51(10):2778–2786
Łaźniewska J, Macioszek VK, Kononowicz AK (2012) Plant-fungus interface: the role of surface structures in plant resistance and susceptibility to pathogenic fungi. Physiol Mol Plant Pathol 78:24–30. https://doi.org/10.1016/j.pmpp.2012.01.004
Novero M, Genre A, Szczyglowski K, Bonfante P (2008) Root hair colonization by mycorrhizal fungi. https://doi.org/10.1007/7089_2008_12
Phillips JM, Hayman DS (1970) Improved procedures for clearing roots and staining parasitic and vesicular-arbuscular mycorrhizal fungi for rapid assessment of infection. Trans Br Mycological Soc 55(1):158–IN18
Prasad A, Shruthi G, Sushma P, Jain AS, Chandan D, Prasad MN (2020) Helicobacter pylori infection: A bioinformatic approach. Int J Pharm Sci Res 11(11):5469–5483. https://doi.org/10.13040/IJPSR.0975-8232.11
Ramlal A, Ahmad S, Kumar L, Khan FN, Chongtham R (2021) From molecules to patients: the clinical applications of biological databases and electronic health records. In: Raza K, Dey N (eds) Translational bioinformatics in healthcare and medicine. Elsevier, Netherlands, pp 107–125
Ramlal A, Samanta A (2022) In Silico functional and phylogenetic analyses of fungal immunomodulatory proteins of some edible mushrooms. AMB Express, 12(1), 159. https://doi.org/10.1186/s13568-022-01503-w
Ramlal A, Harika A, Raju D, Rajendran RA, Lal SK Strigolactones: mediator in abiotic stress responses. In: Raju D, Rajendran RA, Ramlal A, Singh VP (eds) Phytohormones in abiotic stress, pp. 170–182, CRC, Boca Raton
Singh N, Warshel A (2010) Absolute binding free energy calculations: on the accuracy of computational scoring of protein–ligand interactions. Proteins Struct Funct Bioinform 78(7):1705–1723. https://doi.org/10.1002/prot.22687
Trott O, Olson AJ (2010) AutoDockVina: improving the speed and accuracy of Docking with a new scoring function, efficient optimization, and multithreading. J Comput Chem 31(2):455–461. https://doi.org/10.1002/jcc.21334
van Der Heijden MG, Martin FM, Selosse MA, Sanders IR (2015) Mycorrhizal ecology and evolution: the past, the present, and the future. New Phytol 205(4):1406–1423. https://doi.org/10.1111/nph.13288
Yoneyama K, Awad AA, Xie X, Yoneyama K, Takeuchi Y (2010) Strigolactones as germination stimulants for root parasitic plants. Plant Cell Physiol 51(7):1095–1103. https://doi.org/10.1093/pcp/pcq055
Zhang J, Mazur E, Balla J, Gallei M, Kalousek P, Medveďová Z, Friml J (2020) Strigolactones inhibit auxin feedback on PIN-dependent auxin transport canalization. Nat Commun 11(1):3508. https://doi.org/10.1038/s41467-020-17252-y
Author Information
Departemnt of Botany, Midnapore College (Autonomous), Midnapore, India