Therapeutic potential of cyanobacterial protein cyanovirin against monkeypox virus: an in-silico analysis
Sen Gargi, Kar Pallab, Ghosh Sandipan, Roy Ayan, Naidoo Devashan, Sen Arnab
Research Articles | Published: 02 April, 2024
First Page: 1330
Last Page: 1339
Views: 3261
Keywords: Monkeypox virus, Envelope proteins, Cyanovirin, Molecular docking, Molecular dynamics simulations
Abstract
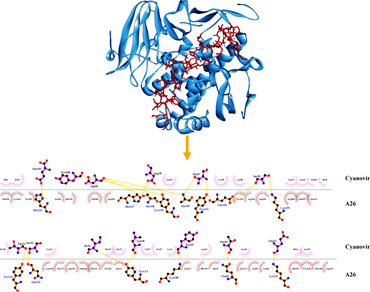
Recent global outbreak of monkeypox virus (MPXV) infections has challenged global public health. Infection cases have been reported from various countries such as North Africa, Middle East, Australia, the Americas, and Europe. Monkeypox virus is zoonotic in the genus Orthopoxvirus that can infect animals as well as human beings. The apprehensions over available therapeutics and vaccines necessitates an immediate need for exploring effective antivirals targeted specifically against MPXV infections. Our study employs extensive molecular docking and molecular dynamics simulations to explore the therapeutic potential of cyanobacterial proteins targeted against envelope proteins of MPXV. AutoDock tools were used to prepare the proteins under study. Molecular docking was executed using PATCHDOCK server. High-score compound was then confirmed using the molecular dynamics simulation for 120 ns using GROMACS ver. 2019 with GROMOS96 43a1 force field parameters. PRODIGY HADDOCK server was used to calculate the binding energies. Dimplot software were used to analyze the protein–protein complexes with lowest binding score and the interactive residues were studied using Ligplot. Our data establish cyanovirin as a promising inhibitor of MPXV envelope proteins A26, A27, D8L, and H3, and generates scope for future in vitro and in vivo studies towards therapeutic development.
References
Abraham MJ, Murtola T, Schulz R, Páll S, Smith JC, Hess B, Lindahl E (2015) GROMACS: high performance molecular simulations through multi-level parallelism from laptops to supercomputers. SoftwareX 1:19–25. https://doi.org/10.1016/j.softx.2015.06.001
Angahar LT (2018) An overview of monkey-pox disease. Am J Curr Microbiol 6(1):39–51. https://doi.org/10.1016/j.amsu.2022.104069
Bartlett JG (2002) Review of literature: general infectious diseases. Infect Dis Clin Pract 11(9):579–582. https://doi.org/10.1097/01.idc.0000090391.89010.d5
Bisht H, Weisberg AS, Moss B (2008) Vaccinia virus L1 protein is required for cell entry and membrane fusion. J Virol 82(17):8687–8694. https://doi.org/10.1128/JVI.00852-08
Bunge EM, Hoet B, Chen L, Lienert F, Weidenthaler H, Baer LR, Steffen R (2022) The changing epidemiology of human monkeypox—a potential threat? A systematic review. PLOS Negl Trop Dis 16(2):0010141. https://doi.org/10.1371/journal.pntd.0010141
Chang TH, Chang SJ, Hsieh FL, Ko TP, Lin CT, Ho MR, Wang AH (2013) Crystal structure of vaccinia viral A27 protein reveals a novel structure critical for its function and complex formation with A26 protein. PLoS Pathog 9(8):1003563. https://doi.org/10.1371/journal.ppat.1003563
Chang HW, Yang CH, Luo YC, Su BG, Cheng HY, Tung SY, Chang W (2019) Vaccinia viral A26 protein is a fusion suppressor of mature virus and triggers membrane fusion through conformational change at low pH. PLoS Pathog 15(6):1007826. https://doi.org/10.1371/journal.ppat.1007826
Chinchar VG (1999) Replication of viruses. Encyclop Virol. https://doi.org/10.1006/rwvi.1999.0245
Ching YC, Chung CS, Huang CY, Hsia Y, Tang YL, Chang W (2009) Disulfide bond formation at the C termini of vaccinia virus A26 and A27 proteins does not require viral redox enzymes and suppresses glycosaminoglycan-mediated cell fusion. J Virol 83(13):6464–6476. https://doi.org/10.1128/JVI.02295-08
Condit RC, Moussatche N, Traktman P (2006) In a nutshell: structure and assembly of the vaccinia virion. Adv Virus Res 66:31–124. https://doi.org/10.1016/S0065-3527(06)66002-8
da Fonseca FG, Wolffe EJ, Weisberg A, Moss B (2000) Effects of deletion or stringent repression of the H3L envelope gene on vaccinia virus replication. J Virol 74(16):7518–7528. https://doi.org/10.1128/jvi.74.16.7518-7528.2000
Datta S, Sarkar I, Goswami N, Mahanta S, Borah P, Sen A (2023) Phytocompounds from Phyllanthus acidus (L.) Skeels in the management of Monkeypox Virus infections. J Biomol Struct Dyn. https://doi.org/10.1080/07391102.2023.2291166
Davies DH, McCausland MM, Valdez C, Huynh D, Hernandez JE, Mu Y, Crotty S (2005) Vaccinia virus H3L envelope protein is a major target of neutralizing antibodies in humans and elicits protection against lethal challenge in mice. J Virol 79(18):11724–11733. https://doi.org/10.1128/JVI.79.18.11724-11733.2005
Dey B, Lerner DL, Lusso P et al (2000) Multiple antiviral activities of Cyanovirin-N: blocking of human immunodeficiency virus type 1 gp120 interaction with CD4 and coreceptor and inhibition of diverse enveloped viruses. J Virol 74:4562–4569. https://doi.org/10.1128/jvi.74.10.4562-4569.2000
Demay J, Bernard C, Reinhardt A, Marie B (2019) Natural products from cyanobacteria: focus on benefificial activities. Mar Drugs 17:320. https://doi.org/10.3390/md17060320
Dittmann E, Gugger M, Sivonen K, Fewer DP (2015) Natural product biosynthetic diversity and comparative genomics of the cyanobacteria. Trends Microbiol 23:642–652. https://doi.org/10.1016/j.tim.2015.07.008
Finlay BB, McFadden G (2006) Anti-immunology: evasion of the host immune system by bacterial and viral pathogens. Cell 124(4):767–782. https://doi.org/10.1016/j.cell.2006.01.034
Howard AR, Senkevich TG, Moss B (2008) Vaccinia virus A26 and A27 proteins form a stable complex tethered to mature virions by association with the A17 transmembrane protein. J Virol 82(24):12384–12391. https://doi.org/10.1128/JVI.01524-08
Huskens D, Schols D (2012) Cyanobacterial lectins as potential HIV microbicide candidates. Mar Drugs 10:1476–1497. https://doi.org/10.3390/md10071476
Jeyaraman M, Selvaraj P, Halesh MB, Jeyaraman N, Nallakumarasamy A, Gupta M, Gupta A (2022) Monkeypox: an emerging global public health emergency. Life 12(10):1590
Johnston JB, McFadden G (2003) Poxvirus immunomodulatory strategies: current perspectives. J Virol 77(11):6093–6100
Kaler J, Hussain A, Flores G, Kheiri S, Desrosiers D (2022) Monkeypox: a comprehensive review of transmission, pathogenesis, and manifestation. Cureus 14(7):26531. https://doi.org/10.7759/cureus.26531
Kar P, Sharma NR, Singh B, Sen A, Roy A (2021) Natural compounds from Clerodendrum spp. as possible therapeutic candidates against SARS-CoV-2: an in silico investigation. J Biomol Struct Dyn 39(13):4774–4785. https://doi.org/10.1080/07391102.2020.1780947
Kar P, Kumar V, Vellingiri B, Sen A, Jaishee N, Anandraj A, Subramaniam MD (2022a) Anisotine and amarogentin as promising inhibitory candidates against SARS-CoV-2 proteins: a computational investigation. J Biomol Struct Dyn 40(10):4532–4542. https://doi.org/10.1080/07391102.2020.1860133
Kar P, Saleh-E-In MM, Jaishee N, Anandraj A, Kormuth E, Vellingiri B, Choi YE (2022b) Computational profiling of natural compounds as promising inhibitors against the spike proteins of SARS-CoV-2 wild-type and the variants of concern, viral cell-entry process, and cytokine storm in COVID-19. J Cell Biochem 123(5):964–986. https://doi.org/10.1002/jcb.30243
Kumla D, Sousa ME, Vasconcelos V, Kijjoa A (2022) Specialized metabolites from cyanobacteria and their biological activities. In: The pharmacological potential of cyanobacteria. Academic Press, pp 21–54
Lin CL, Chung CS, Heine HG, Chang W (2000) Vaccinia virus envelope H3L protein binds to cell surface heparan sulfate and is important for intracellular mature virion morphogenesis and virus infection in vitro and in vivo. J Virol 74(7):3353–3365. https://doi.org/10.1128/jvi.74.7.3353-3365.2000
Loo YM, Gale Jr M (2007) Viral regulation and evasion of the host response. Interferon 50th Anniv 295–313
Magnus PV, Andersen EK, Petersen KB, Birch-Andersen A (1959) A pox-like disease in cynomolgus monkeys. Acta Pathol Microbiol Scand A 46(2):156–176. https://doi.org/10.1111/j.1699-0463.1959.tb00328.x
Matho MH, Maybeno M, Benhnia MREI, Becker D, Meng X, Xiang Y, Zajonc DM (2012) Structural and biochemical characterization of the vaccinia virus envelope protein D8 and its recognition by the antibody LA5. J Virol 86(15):8050–8058. https://doi.org/10.1128/JVI.00836-12
Mcfeeters RL, Xiong C, O’Keefe BR et al (2007) The novel fold of scytovirin reveals a new twist for antiviral entry inhibitors. J Mol Biol 369:451–461. https://doi.org/10.1016/j.jmb.2007.03.030
Moss B (1996) Genetically engineered poxviruses for recombinant gene expression, vaccination, and safety. Proc Natl Acad Sci USA 93(21):11341–11348. https://doi.org/10.1073/pnas.93.21.11341
Moss B (2012) Poxvirus cell entry: how many proteins does it take? Viruses 4(5):688–707. https://doi.org/10.3390/v4050688
Moss B (2016) Membrane fusion during poxvirus entry. Semin Cell Dev Biol 60:89–96
Muñoz-Basagoiti J, Monteiro FLL, Krumpe LR, Armario-Najera V, Shenoy SR, Perez-Zsolt D, O’Keefe BR (2023) Cyanovirin-N binds to select SARS-CoV-2 spike oligosaccharides outside of the receptor binding domain and blocks infection by SARS-CoV-2. Proc Natl Acad Sci 120(10):e2214561120
Murugan T (2011) Screening for antifungal and antiviral activity of C-phycocyanin from Spirulina platensis. J Pharm Res 4:4161–4163
Naidoo D, Roy A, Kar P, Mutanda T, Anandraj A (2020) Cyanobacterial metabolites as promising drug leads against the Mpro and PLpro of SARS-CoV-2: an in silico analysis. J Biomol Struct Dyn 39(16):6218–6230. https://doi.org/10.1080/07391102.2020.1794972
Naidoo D, Kar P, Roy A, Mutanda T, Bwapwa J, Sen A, Anandraj A (2021) Structural insight into the binding of cyanovirin-N with the spike glycoprotein, Mpro and PLpro of SARS-CoV-2: protein–protein interactions, dynamics simulations and free energy calculations. Molecules 26(17):5114. https://doi.org/10.3390/molecules26175114
Otu A, Ebenso B, Walley J, Barceló JM, Ochu CL (2022) Global human monkeypox outbreak: atypical presentation demanding urgent public health action. Lancet Microbe 3(8):554-e555. https://doi.org/10.1016/S2666-5247(22)00153-7
Sánchez-Puig JM, Sánchez L, Roy G, Blasco R (2004) Susceptibility of different leukocyte cell types to vaccinia virus infection. Virol J 1(1):1–7. https://doi.org/10.1186/1743-422X-1-10
Schneidman-Duhovny D, Inbar Y, Nussinov R, Wolfson HJ (2005) PatchDock and SymmDock: servers for rigid and symmetric docking. Nucl Acids Res 33:363–367. https://doi.org/10.1093/nar/gki481
Schwede T, Kopp J, Guex N, Peitsch MC (2003) SWISS-MODEL: an automated protein homology-modeling server. Nucl Acids Res 31(13):3381–3385. https://doi.org/10.1093/nar/gkg520
Simpson K, Heymann D, Brown CS, Edmunds WJ, Elsgaard J, Fine P, Jones TC (2020) Human monkeypox—after 40 years, an unintended consequence of smallpox eradication. Vaccine 38(33):5077–5081. https://doi.org/10.1016/j.vaccine.2020.04.062
Singh K, Gittis AG, Gitti RK, Ostazeski SA, Su HP, Garboczi DN (2016) The vaccinia virus H3 envelope protein, a major target of neutralizing antibodies, exhibits a glycosyltransferase fold and binds UDP-glucose. J Virol 90(10):5020–5030. https://doi.org/10.1128/JVI.02933-15
Stanford MM, McFadden G, Karupiah G, Chaudhri G (2007) Immunopathogenesis of poxvirus infections: forecasting the impending storm. Immunol Cell Biol 85(2):93–102. https://doi.org/10.1038/sj.icb.7100033
Trott O, Olson AJ (2010) AutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J Comput Chem 31(2):455–461. https://doi.org/10.1002/jcc.21334
Tovchigrechko A, Vakser IA (2006) GRAMM-X public web server for protein-protein docking. Nucl Acids Res 34:310–314. https://doi.org/10.1093/nar/gkl206
Vangone A, Bonvin AM (2017) PRODIGY: a contact-based predictor of binding affinity in protein-protein complexes. Bio Protocol 7(3):2124–2124. https://doi.org/10.21769/BioProtoc.2124
Wallace AC, Laskowski RA, Thornton JM (1995) LIGPLOT: a program to generate schematic diagrams of protein–ligand interactions. Protein Eng Des Sel 8(2):127–134. https://doi.org/10.1093/protein/8.2.127
Author Information
Bioinformatics Facility Centre, University of North Bengal, Siliguri, India