A mathematical model to elucidate of photosynthetic apparatus in noni (Morinda citrifolia L.) to temperature stress
Research Articles | Published: 21 September, 2023
First Page: 2339
Last Page: 2346
Views: 3130
Keywords: Mathematical model, Noni, Photosynthetic pigments, Temperature, Electron transport chain
Abstract
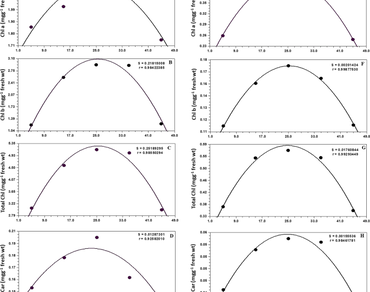
This study aimed to develop a mathematical model to understand the acclimatization potential of photosynthetic electron transport in both tissue of leaf and developing fruit, exposed to contrasting temperatures. This dynamic mathematical model covers photosynthetic apparatus that contain variety of pigments, photo-systems, electron carriers, and final electron acceptor complex, ferredoxin. The proposed model explained the variations of photosynthetic pigments pool viz., Chla, Chlb, total Chlorophyll, and Carotenoids; OJIP transients i.e., Fv/Fo, Fv/Fm, φ(Eo), Plabs and Pltotal concerning varied temperature in a perfect way with r = ~ 1 and S = ~ 0 for leaves as well as developing fruits. Since the correlation coefficients for the parameters are unity, perfect validity of the hypothesis and developed model. The developed model could be quite useful for accounting total associated physiological responses and for determining optimum temperature requirements for the healthy performance of plants. This model will be used to develop strategies for better growth and development of Noni plants in the current scenario of global climate change.
References
Adams RP (2001) Identification of essential oil components by gas chromatography/quadrupole mass spectroscopy. Allured Publishing Co, Carol Stream
Boontha S, Buranrat B, Pitaksuteepong T (2020) Cytotoxic and anti-migratory effects on Michigan cancer foundation-7 cells of Morinda citrifolia L. leaf extract and formulation of tablets from the extract. Phcog Res 12:24–28
Chen S, Yang J, Zhang M, Strasser RJ, Qiang S (2016) Classification and characteristics of heat tolerance in Ageratina adenophora populations using fast chlorophyll a fluorescence rise O-J-I-P. Environ Exp Bot 122(1):126–140. https://doi.org/10.1016/j.envexpbot.2015.09.011
Demetriou G, Neonaki C, Navakoudis E, Kotzabasis K (2007) Salt stress impact on the molecular structure and function of the photosynthetic apparatus—the protective role of polyamines. Biochim Biophys Acta (BBA) Bioenerget 1767(4):272–280
Dutta S, Mohanty S, Tripathy BC (2009) Role of temperature stress on chloroplast biogenesis and protein import in pea. Plant Physiol 150:1050–1061. https://doi.org/10.1104/pp.109.137265
Fan J, Hu Z, Xie Y, Chan Z, Chen K, Amombo E, Chen L, Fu J (2015) Alleviation of cold damage to photosystem II and metabolisms by melatonin in Bermudagrass. Front Plant Sci 6:925
Govindjee (2004) Chlorophyll a fluorescence: a bit of basic and history. In: Papageorgiou GC, Govindjee (eds) Chlorophyll a fluorescence: a signature of photosynthesis, advances in photosynthesis and respiration, vol 19. Springer, Dordrecht, pp 1–41
Gupta R (2019) Tissue-specific disruption of photosynthetic electron transport rate pigeon pea (Cajanuscajan L.) under elevated temperature. Plant Signal Behav 14:1601952
Gupta R (2020a) The oxygen-evolving complex: a super catalyst for life on earth, in response to abiotic stresses. Plant Signal Behav 15(12):1824721. https://doi.org/10.1080/15592324.2020.1824721
Gupta R (2020b) Manganese repairs the oxygen-evolving complex (OEC) in maize (Zea mays L.) damage during seawater vulnerability. J Soil Sci Plant Nutr 20:1387–1396. https://doi.org/10.1007/s42729-020-00220-2
Gupta R, Sharma RD, Rao YR, Siddiqui ZH, Verma A, Ansari MW, Rakwal R, Tuteja N (2020a) Acclimation potential of Noni (Morinda citrifolia L.) plant to temperature stress is mediated through photosynthetic electron transport rate. Plant Signal Behav. https://doi.org/10.1080/15592324.2020.1865687
Gupta R, Sharma RD, Singh M (2020b) Energy dissipation and photosynthetic electron flow during the transition from juvenile red to mature green leaves in mango (Mangifera indica L.). Plant Biosyst. https://doi.org/10.1080/11263504.2020.1810807
Kalaji HM, Rastogi A, Živčák M, Brestic M, Daszkowska-Golec A, Sitko K, Alsharafa KY, Lotfi R, Stypiński P, Samborska IA, Cetner MD (2018) Prompt chlorophyll fluorescence as a tool for crop phenotyping: an example of barley landraces exposed to various abiotic stress factors. Photosynthetica 56:953–961
Killi D, Bussotti F, Gottardini E, Pollastrini M, Mori J, Tani C, Papini A, Ferrini F, Fini A (2018) Photosynthetic and morphological responses of oak species to temperature and [CO2] increased to levels predicted for 2050. Urban for Urban Green 31:26–37
Kirk JTO, Allen RL (1965) Dependence of chloroplast pigments synthesis on protein synthetic effects on the action. Biochem Biophys Res J Can 27:523–530
Lazar D (2006) The polyphasic chlorophyll a fluorescence rise measured under high intensity of exciting light. Funct Plant Biol 33:9–30
Mahantesh PS, Hiremath JS, Lokesh CH, Ravi Y, Sameer-Hussain MD, Pooja MR (2018) Noni a wonder plant (therapeutic properties): a review. Int J Curr Microbiol App Sci 7:2722–2728. https://doi.org/10.20546/ijcmas.2018.702.331
Malik AR, Dar BN, Wani SH (2009). Noni (Morinda citrifolia L.): hope in a bottle. In: New biology: current developments/frontiers in life sciences, pp 340–351
Mohanty S, Baishna BG, Tripathy C (2006) Light and dark modulation of chlorophyll biosynthetic genes in response to temperature. Planta 224:692–699. https://doi.org/10.1007/s00425-006-0248-6
Ojeda-Pérez ZZ, Jiménez-Bremont JF, Delgado-Sánchez P (2017) Continuous high and low temperature induced a decrease of photosynthetic activity and changes in the diurnal fluctuations of organic acids in Opuntia streptacantha. PLoS ONE 12:e0186540
Papageorgiou GC, Govindjee (eds) (2004) Chlorophyll a fluorescence: a signature of photosynthesis. Advances in photosynthesis and respiration, vol 19. Springer, Dordrecht, p 820
Porra RJ (2002) The chequered history of the development and use of simultaneous equations for the accurate determination of chlorophylls a and b. Photosynth Res 73:149–156
Preston JC, Sandve SR (2013) Adaptation to seasonality and the winter freeze. Front Plant Sci 4:167
Rachmilevitch S, DaCosta M, Huang B (2006) Physiological and biochemical indicators for stress tolerance, plant–environment interactions, 3rd edn. CRC Press, Boca Raton, pp 321–356
Stefanov D, Petkova V, Denev ID (2011) Screening for heat tolerance in common bean (Phaseolus vulgaris L.) lines and cultivars using JIP-test. Sci Hortic 128:1–6
Stirbet A, Lazár D, Kromdijk J, Govindjee (2018) Chlorophyll a fluorescence induction: can just a one-second measurement be used to quantify abiotic stress responses? Photosynthetica. https://doi.org/10.1007/s11099-018-0770-3
Strasser RJ, Tsimilli-Michael M, Srivastava A (2004) Analysis of the chlorophyll a fluorescence transient. In: Papadogeorgiou GC, Govindjee T (eds) chlorophyll a fluorescence: a signature of photosynthesis. Springer, The Netherlands, pp 321–362
Strasser RJ, Tsimilli-Michael M, Qiang S, Goltsev V (2010) Simultaneous in vivo recording of prompt and delayed fluorescence and 820-nm reflection changes during drying and after rehydration of the resurrection plant Haberlea rhodopensis. Biochim Biophys Acta 1797:1313–1326
Twardosz R, Kossowska-Cezak U (2015) Exceptionally cold and mild winters in Europe (1951–2010). Theor Appl Climatol 125:1–13
Wong C (2020) Health benefits of noni juice: Polynesian folk remedy poses certain health risks. https://www.verywellfit.com/noni-juice-what-you-need-to-know-88326. Accessed 20 July 2020
Xiaoli Wu, Tang Y, Li C, Chun Wu, Huang G (2015) Chlorophyll fluorescence and yield responses of winter wheat to waterlogging at different growth stages. Plant Prod Sci 18(3):284–294. https://doi.org/10.1626/pps.18.284
Zushi K, Kajiwara S, Matsuzoe N (2012) Chlorophyll a fluorescence OJIP transient as a tool to characterize and evaluate response to heat and chilling stress in tomato leaf and fruit. Sci Hortic 148:39–46
Author Information
Department of Biology, Faculty of Natural Sciences, The University of Guyana, Georgetown, Guyana