Micropropagation of Vanilla planifolia Jacks. RH330 genotypes resistant to Fusarium oxysporum f. sp. vanillae in double-phase culture
*Article not assigned to an issue yet
Fernández-Villa Zelzin Eréndira, Iglesias-Andreu Lourdes Georgina
Research Articles | Published: 25 April, 2024
Online ISSN : 2229-4473.
Website:www.vegetosindia.org
Pub Email: contact@vegetosindia.org
First Page: 0
Last Page: 0
Views: 163
Keywords:
Elongation, Rooting, Phenolization, Hyperhydricity, Gibberellic acid
Abstract
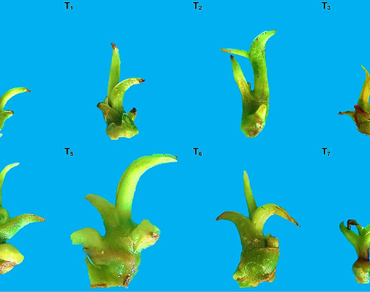
A highly valuable species, Vanilla planifolia Jacks., is threatened with extinction due to its extreme susceptibility to Fusarium oxysporum f. sp. vanillae (Fov). It is therefore essential to propagate genetic material such as the RH330 genotype, selected for its in vitro resistance to 30% of the pathogenic strain H3 of Fov fungal filtrates. For this reason, this work was developed with the aim of evaluating the effect of a double-phase culture (DPC) system in relation to a semi-solid medium (SSM) culture system, as well as different concentrations of GA3 on hypocotyl elongation and rooting of shoots of V. planifolia RH330. A total of 8 treatments of 15 shoots each: T0-T3 semi-solid MS medium cultures with four concentrations of GA3 (0, 0.5, 1, and 1.5 mg L− 1), and T4-T7 double-phase cultures with four concentrations of GA3 (0, 0.5, 1, and 1.5 mg L− 1), in their liquid phase, were evaluated in a completely randomized design. After eight weeks, hypocotyl elongation, the number of leaves and roots, and root length were evaluated. The treatment of the DPC system without the addition of GA3 (T4) showed the greatest hypocotyl elongation (0.36 cm), while the treatment of the DPC system with the addition of 1.5 mg L− 1 GA3 in the liquid phase (T7) produced the greatest number of leaves (1.05 cm). The highest number and length of roots (1.91 and 2.67 cm, respectively) were obtained in the control treatment. Compared to the traditional method of propagation in semi-solid culture medium (SSC), the DPC system without GA3 was the most successful in producing seedlings with the desired characteristics, such as longer hypocotyl length and the presence of longer roots.
(*Only SPR Members can get full access. Click Here to Apply and get access)
References
Abebe Z, Mengesha A, Teressa A, Tefera W (2009) Efficient in vitro multiplication protocol for Vanilla planifolia using nodal explants in Ethiopia. Afr J Biotechnol 8(24):6817–6821
Alcántara-Cortes JS, Acero-Godoy J, Alcántara-Cortés JD, Sánchez-Mora RM (2019) Principales reguladores hormonales y sus interacciones en El Crecimiento Vegetal. NOVA 17(32):109–129
Amente G, Chimdessa E (2021) Control of browning in plant tissue culture: a review. J Sci Agric 5:67–71. https://doi.org/10.25081/jsa.2021.v5.7266
Andrade-Andrade G, Delgado-Alvarado A, Herrera-Cabrera BE, Arévalo-Galarza L, Caso-Barrera L (2018) Variación de compuestos fenólicos totales, flavonoides y taninos en Vanilla planifolia Jacks. ex Andrews de la Huasteca Hidalguense, México. Agrociencia 52(1): 55–66
CITES (2019) Convención Sobre el Comercio Internacional de Especies Amenazadas de Fauna y Flora Silvestres. Vanilla planifolia. Accessed from: https://cites.org/eng/taxonomy/term/40959
da Silva IAO, Biasi LA (2022) Double-phase culture medium and plant growth regulators in the micropropagation of blackberries. Comunicata Sci 13:e3613. https://doi.org/10.14295/CS.v13.3613
de Oliveira SOD, Sayd RM, Balzon TA, Scherwinski-Pereira JE (2013) A new procedure for in vitro propagation of vanilla (Vanilla planifolia) using a double-phase culture system. Sci Hort 161:204–209. https://doi.org/10.1016/j.scienta.2013.06.039
Erawati DN, Wardati I, Humaida s, Mawadah Y, Ikanafi’ah A, Ryana WM (2021) Shoots multiplication of vanilla (Vanilla planifolia) with benzyl amino purine and kinetin modification. IOP Conf Series: Earth Environ Sci 672:012007. https://doi.org/10.1088/1755-1315/672/1/012007
García-Bofill M, Sutton PW, Straatman H, Brummund J, Schürmann M, Guillén M, Álvaro G (2021) Biocatalytic synthesis of vanillin by an immobilised eugenol oxidase: high biocatalyst yield by enzyme recycling. Appl Catal A 610:117934. https://doi.org/10.1016/j.apcata.2020.117934
Jerald S, Gangaprasad A, Mathew SP (2023) Double phase culture system mediated enhances protocol for shoor proliferation of Vanilla Andamanica Rolfe.– an endemic wild relative of commercial Vanilla from the Andaman and Nicobar Islands. Plant Sci Today 10(4):186–191. https://doi.org/10.14719/pst.2475
López C, Suárez I (2018) In vitro arrow cane (Gynerium Sagitatum Aubl.) Multiplication in double phase medium. Revista De Ciencias Agrícolas 35(2):5–13. https://doi.org/10.22267/rcia.183502.86
Mahardhini AD, Anwar S, Karno (2023) BAP and kinetin application for in vitro bud induction of vanilla (Vanilla planifolia Andrews.) From stem node explants in the initiation stage. IOP Conf Series: Earth Environ Sci 1246:012030. https://doi.org/10.1088/1755-1315/1246/1/012030
Mahmoud LM, Killiny N, Dutt M (2024) Melatonin supplementation enhances browning suppression and improves transformation efficiency and regeneration of transgenic rough lemon plants (Citrus × jambhiri). PLoS ONE 19(3):e0294318. https://doi.org/10.1371/journal.pone.0294318
Malhotra EV, Bansal S, Pathania P, Mali SC, Agrawal A (2021) Regeneration of plantlets through protocorm-like bodies: an alternative method for vanilla micro propagation. Indian J Biotechnol 20(4):388–395
Mosquera-Espinosa AT, Bonilla-Monar A, Flanagan NS, Rivas Á, Sánchez F, Chavarriaga P, Bedoya A, Riascos-Ortiz D (2022) In vitro evaluation of the development of Fusarium in vanilla accessions. Agronomy 12(11): 2831. https://doi.org/10.3390/agronomy12112831
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15(3):473–497. http://doi.wiley.com/https://doi.org/10.1111/j.1399-3054.1962.tb08052.x
Nagai K, Mori Y, Ishikawa S, Furuta T, Gamuyao R, Niimi Y, Hobo T, Fukuda M, Kojima M, Takebayashi Y, Fukushima A, Himuro Y, Kobayashi M, Ackley W, Hisano H, Sato K, Yoshida A, Wu J, Wu J, Sakakibara H, Sato Y, Tsuji H, Akagi T, Ashikari M (2020) Antagonistic regulation of the gibberellic acid response during stem growth in rice. Nature 584(7819):109–114. https://doi.org/10.1038/s41586-020-2501-8
Ortega-Macareno LC, Iglesias-Andreu LG, Luna-Rodríguez M, Noa-Carrazana JC (2023) Resistance induction in Vanilla planifolia jacks. By foliar spray of salicylic acid (SA) against Fusarium oxysporum f. sp. vanillae. https://doi.org/10.1007/s42535-023-00740-z. Vegetos
Pinaria AG, Laurence MH, Burgess LW, Liew ECY (2015) Phylogeny and origin of Fusarium oxysporum f. sp. vanillae in Indonesia. Plant Pathol 64(6):1358–1365. https://doi.org/10.1111/ppa.12365
Polivanova OB, Bedarev VA (2022) Hyperhydricity in plant tissue culture. Plants 11(23):3313. https://doi.org/10.3390/plants11233313
Ramírez-Mosqueda MA, Iglesias-Andreu LG (2016) Evaluation of different temporary immersion systems (BIT®, BIG, and RITA®) in the micropropagation of Vanilla planifolia Jacks. Vitro Cell Dev Biology-Plant 52(2):154–160. https://doi.org/10.1007/s11627-015-9735-4
Ramírez-Mosqueda MA, Iglesias-Andreu LG, Teixeira-da Silva JA, Luna-Rodríguez M, Noa-Carrazana JC, Bautista-Aguilar JR, Leyva-Ovalle OR, Murguía-González J (2019) In vitro selection of vanilla plants resistant to Fusarium oxysporum f. sp. vanillae. Acta Physiol Plant 41(40): (2019). https://doi.org/10.1007/s11738-019-2832-y
Ramírez-Mosqueda MA, Iglesias-Andreu LG, Luna-Rodríguez M, Castro-Luna AA (2015) In vitro phytotoxicity of culture filtrates of Fusarium oxysporum f. sp. vanillae in Vanilla planifolia Jacks. Scientia Horticulturae 197: 573–578. https://doi.org/10.1016/j.scienta.2015.10.019
Ríos-Barreto Y, Arellano-Ostoa G, Fernández-Pavía YL, García-Villanueva E, Tejeda-Sartorius O (2023) Plant growth and early in vitro floral differentiation of vanilla (Vanilla planifolia jacks. Ex Andrews). Agro Productividad 16(1):127–135. https://doi.org/10.32854/agrop.v16i1.2439
Rodrigues LA, Paiva-Neto VBD, Boaretto AG, Oliveira JFD, Torrezan MDA, Lima SFD, Otoni WC (2015) In vitro propagation of Cyrtopodium Saintlegerianum rchb. f. (orchidaceae), a native orchid of the Brazilian savannah. Crop Breed Appl Biotechnol 15:10–17. https://doi.org/10.1590/1984-70332015v15n1a2
San-José MC, Blázquez N, Cernadas MJ, Janeiro LV, Cuenca B, Sánchez C, Vidal N (2020) Temporary immersion systems to improve alder micropropagation. Planr Cell Tissue Organ Cult 143:265–275. https://doi.org/10.1007/s11240-020-01937-9
SEMARNAT (2010) Norma Oficial Mexicana NOM 059-SEMARNAT-2010. Protección ambiental-Especies nativas de México de flora y fauna silvestres-Categorías de riesgo y especificaciones para su inclusión, exclusión o cambio-Lista de especies en riesgo. Accessed from: https://www.dof.gob.mx/normasOficiales/4254/semarnat/semarnat.htm
Senapati SK (2015) A double phase culture system: an economic and time saving protocol for in vitro propagation of plant. Scholarena J Biotechnol 2(1):1–5. https://doi.org/10.18875/2375-6713.1.301
Sota V, Benelli C, Cuko B, Kongjika E (2021) Effect of a double phase culture system and activated charcoal on in vitro propagation of Malus sylvestris (L.) Mill. Adv Hortic Sci 35(4):361–369. https://doi.org/10.36253/ahsc-11825
Suares-Padrón IE, Pérez-Meza PM, López-Díaz CM (2020) Evaluation of sucrose and GA3 in an in vitro shoot culture of Alpinia purpurata (Zingiberaceae). Ciencia Y Tecnología Agropecuaria 21(2):e1193. https://doi.org/10.21930/rcta.vol21_num2_art:1193
Vásquez-Hernández S, Cruz-Cruz CA, Santiago-Santiago M, Bello-Bello JJ (2020) Evaluation of different antioxidants during in vitro establishment of allspice (Pimenta dioica L. Merrill): a recalcitrant species. Res Square. https://doi.org/10.21203/rs.3.rs-21190/v1
Acknowledgements
Author Information
Instituto de Biotecnología y Ecología Aplicada (INBIOTECA), Universidad Veracruzana, Xalapa, Mexico
zelzinfv@gmail.com
Instituto de Biotecnología y Ecología Aplicada (INBIOTECA), Universidad Veracruzana, Xalapa, Mexico
liglesias@uv.mx