Bio-nematicidal activities by culture filtrate of Bacillus subtilis HussainT-AMU: new promising biosurfactant bioagent for the management of Root Galling caused by Meloidogyne incognita
Hussain Touseef, Haris Mohammad, Shakeel Adnan, Ahmad Gufran, Ahmad Khan Abrar, Khan Mohd. A.
Research Articles | Published: 27 January, 2020
First Page: 229
Last Page: 238
Views: 4187
Keywords: Biocontrol, Biosurfactant, Root knot nematode, Bacillus subtilis
Abstract
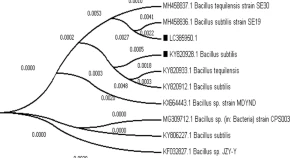
Root galling caused by Meloidogyne incognita causes dramatic yield reduction in a wide range of crops, especially vegetables, at most 15–25%, however, in severe cases it can reach upto 75%. A new biosurfactant bacteria characterized through 16S rRNA gene sequencing and biochemically as Bacillus subtilis HussainT-AMU that was isolated from oil contaminated soil from Odisha coat line near Paradip port exhibiting significant biosurfactant activity in all screening assays. Most of the in vitro experiments were carried out at room temperature. Exposure of fresh culture filtrate from new bioagent to M. incognita caused substantial mortality of the second stage juvenile (J2) and significantly reduced egg hatchability. 100% of culture filtrate collected from B. subtilis HussainT-AMU caused J2 mortality and reduced hatching (p < 0.05). Hatching was defective by 97–99%. More than 90% J2 mortality occurred after 24 h of exposure in 100%. Significant reduction (58.79%) in number of galls/plant in tomato roots in combined application leading to reduction in root galling was recorded. This novel biosurfactant bacterial strain seems to be a safe and effective bio-nematicide potential that needs to be explored more both at field study as well genomic level.
References
- Aalten PM, Vitour D, Blanvillain D, Gowen SR, Sutra L (1998) Effect of rhizosphere fluorescent Pseudomonas isolates on plant parasitic nematodes Radopholus similis and Meloidogyne spp. Lett Appl Microbiol 27:357–361
- Abbasi M, Ahmed N, Zaki M, Shuakat S, Khan D (2014) Potential of Bacillus species against Meloidogyne javanica parasitizing eggplant (Solanum melongena L.) and induced biochemical changes. Plant Soil 375:159–173
- Ali NI, Siddiqui IA, Shahid Shaukat S, Zaki MJ (2002) Nematicidal activity of some strains of Pseudomonas spp. Soil Biol Biochem 34:1051–1058
- Atibalentja N, Noel GR, Domier LL (2000) Phylogenetic position of the North American isolate of Pasteuria that parasitizes the soybean cyst nematode, Heterodera glycines, as inferred from 16S rDNA sequence analysis. Int J Syst Evol Microbiol 50:605–613
- Babu S, Seetharaman K, Nandakumar R (2004) Biocontrol efficacy of Pseudomonas fluorescens against Alternaria solani and tomato leaf blight disease. Ann Appl Sci Res 5(4):392–403
- Bais HP, Fall R, Vivanco JM (2004) Biocontrol of Bacillus subtilis against infection of Arabidopsis roots by Pseudomonas syringae is facilitated by biofilm formation and surfactin production. Plant Physiol 134:307–319
- Basyony AG, Abo-Zaid GA (2018) Biocontrol of the root-knot nematode, Meloidogyne incognita, using an eco-friendly formulation from Bacillus subtilis, lab. and greenhouse studies. Egypt J Biol Pest Control 28:87. https://doi.org/10.1186/s41938-018-0094-4
- Becker JO, Zavaleta-Mejia E, Colbert SF, Schroth MN, Weinhold AR, Hancock GJ, Van Gundy SD (1988) Effects of rhizobacteria on root-knot nematodes and gall formation. Phytopathology 78:1466–1469
- Bhattacharjee RB, Jourand P, Chaintreuil C, Dreyfus B, Singh A, Mukhopadhyay SN (2012) Indole acetic acid and ACC deaminase-producing Rhizobium leguminosarum bv. trifolii SN10 promote rice growth, and in the process undergo colonization and chemotaxis. Biol Fertil Soils 48:173–182
- Danhorn T, Fuqua C (2007) Biofilm formation by plant-associated bacteria. Annu Rev Microbiol 61:401–422
- Degenkolb T, Vilcinskas A (2016) Metabolites from nematophagous fungi and nematicidal natural products from fungi as an alternative for biological control. Part I: metabolites from nematophagous ascomycetes. Appl Microbiol Biotechnol 100(9):3799–3812
- Djordjevic D, Wiedmann M, McLandsborough LA (2002) Microtiter plate assay for assessment of Listeria monocytogenes biofilm formation. Appl Environ Microbiol 68:2950–2958
- Ebadi M, Fatemy S, Riahi H (2009) Evaluation of Pochonia chlamydosporia var. chlamydosporia as a control agent of Meloidogyne javanica on pistachio. Biocontrol Sci Technol 19:689–700
- El-Hadad ME, Mustafa MI, Selim ShM, El-Tayeb TS, Mahgoob AEA, Aziz NHA (2011) The nematicidal effect of some bacterial biofertilizers on Meloidogyne incognita in sandy soil. Braz J Microbiol 42:105–113
- El-Nagdi WMA, Youssef MMA (2004) Soaking faba bean seed in some bioagents as prophylactic treatment for controlling Meloidogyne incognita root-knot nematode infection. J Pest Sci 77:75–78
- Engelbrecht G, Horak I, Peet J, van Rensburg J, Claassens S (2018) Bacillus-based bionematicides: development, modes of action and commercialisation. Biocontrol Sci Technol 28:7629–7653. https://doi.org/10.1080/09583157.2018.1469000
- Fourie H, Jones VW, Daneel RK, De Waele D (2017) Nematology in South Africa: a view from the 21st century. Springer International, Cham
- Gao H, Qi G, Yin R, Zhang H, Li C, Zhao X (2016) Bacillus cereus strain S2 shows high nematicidal activity against Meloidogyne incognita by producing sphingosine. Sci Rep 6:605
- Haegeman A, Mantelin S, Jones JT, Gheysen G (2012) Review: functional roles of effectors of plant-parasitic nematodes. Gene 492:19–31
- Hartman KM, Sasser JN (1985) Identification of Meloidogyne species on the basis of differential host test and perineal-pattern morphology. In: Barker KR, Carter CC, Sasser JN (eds) An advanced treatise on Meloidogyne, methodology, vol 2. North Carolina State University Graphics, Raleigh, pp 69–76
- Hashem M, Abo-Elyousr KA (2011) Management of the root-knot nematode Meloidogyne incognita on tomato with combinations of different biocontrol organisms. Crop Prot 30:285–292
- Holajjer P, Dey R, Pal KK, Chakraborty K, Harish G, Nataraja MV, Deepak E (2018) Assessment of nematicidal properties of fluorescent pseudomonads using peanut root-knot nematode, Meloidogyne arenaria. J Biol Control 32(3):193–202
- Huang XW, Tian BY, Niu QH, Yang JK, Zhang LM et al (2005) An extracellular protease from Brevibacillus laterosporus G4 without parasporal crystals can serve as a pathogenic factor in infection of nematodes. Res Microbiol 156:719–727
- Huang Y, Xu CK, Ma L, Zhang KQ, Duan CQ, Mo MH (2010) Characterisation of volatiles produced from Bacillus megaterium YFM3.25 and their nematicidal activity against Meloidogyne incognita. Eur J Plant Pathol 126:417–422
- Hussain T, Khan AA (2019) Biological control of black scurf of potato caused by Rhizoctonia solani using lipopeptides produced by noval biosurfactant bacteria: Bacillus subtilis Hussain T-AMU isolated from Paradip port, Odisha, India. In: National conference on biointensive approaches in plant protection strategies and their socio-economic impacts held on 29th–30th Oct. 2018 at Dept. of Plant Protection, Aligarh Muslim University, Aligarh, India under Indian Phytopathological society, Mid-Eastern Zone region annual meeting. OP, p 31
- Hussain T, Khan AA (2020) Bacillus subtilis HussainT-AMU and its antifungal activity against Potato black scurf caused by Rhizoctonia solani. Biocatal Agric Biotechnol 23:101433
- Hussey RS, Barker KR (1973) A comparison of methods of collecting inocula of Meloidogyne spp., including a new technique. Plant Dis Report 57:1025–1028
- Karuri HW, Olago D, Neilson R, Mararo E, Villinger J (2017) A survey of root knot nematodes and resistance to Meloidogyne incognita in sweet potato varieties from Kenyan fields. Crop Prot 92:114–121
- Khan Z, Park SD, Shin SY, Bae SG, Yeon IK, Seo YJ (2005) Management of Meloidogyne incognita on tomato by root-dip treatment in culture filtrate of the bluegreen alga, Microcoleus vaginatus. Bioresour Technol 96:1338–1341
- Kim D, Cook J, Weller D (1997) Bacillus sp. L324-92 for biological control of three root diseases of wheat grown with reduced tillage. Phytopathology 87:551–558
- Kloepper JW, Ryu CM, Zhang SA (2004) Induced systemic resistance and promotion of plant growth by Bacillus sp. Phytopathology 94:1259–1266
- Kuiper I, Lagendijk EL, Pickford R, Derrick JP, Lamers GEM, Thomas-Oates JE, Lugtenberg BJJ, Bloemberg GV (2004) Characterization of two Pseudomonas putida lipopeptide biosurfactants, putisolvin I and II, which inhibit biofilm formation and break down existing biofilms. Mol Microbiol 51:97–113
- Mendoza AR, Kiewnick S, Sikora RA (2008) In vitro activity of Bacillus firmus against the burrowing nematode Radopholus similis, the root-knot nematode Meloidogyne incognita and the stem nematode Ditylenchus dipsaci. Biocontrol Sci Technol 18:377–389
- Moryl M, Spętana M, Dziubek K, Paraszkiewicz K, Różalska S, Płaza GA, Różalski A (2015) Antimicrobial, antiadhesive and antibiofilm potential of lipopeptides synthesised by Bacillus subtilis, on uropathogenic bacteria. Acta Biochim Pol 62(4):725–732
- Neipp PW, Becker JO (1999) Evaluation of biocontrol activity of rhizobacteria from Beta vulgaris against Heterodera schachtii. J Nematol 31:54–61
- Nitschke M, Costa SGVAO (2007) Biosurfactant in food industry. Trends Food Sci Technol 18:252–259
- Niu Q, Huang X, Zhang L, Lian L, Li Y, Li J, Zhang K (2007) Functional identification of the gene bace16 from nematophagous bacterium Bacillus nematocida. Appl Microbiol Biotechnol 75:141–148
- Ongena M, Jacques P (2008) Bacillus lipopeptides: versatile weapons for plant disease biocontrol. Trends Microbiol 16:115–125
- Onkendi EM, Kariuki GM, Marais M, Moleleki LN (2014) The threat of root-knot nematodes (Meloidogyne spp.) in Africa: a review. Plant Pathol 63(4):727–737
- Perneel M, Dhondt L, De Maeyer K, Adiobo A, Rabey K, Hofte M (2008) Phenazines and biosurfactants interact in the biological control of soil-borne diseases caused by Phythium spp. Environ Microbiol 10:778–788
- Raini R, Hoffmann Vand Zebitz V, Zebitz CPW (2005) Integrated Pest Management (IPM) and information flow: case study tomato stakeholders’ practices in Kenya. Tropentag, Stuttgart, pp 1–4
- Ramezani Moghaddam M, Mahdikhani Moghaddam E, Baghaee Ravari S, Rouhani H (2014) The nematicidal potential of local Bacillus species against the root-knot nematode infecting greenhouse tomatoes. Biocontrol Sci Technol 24(3):279–290. https://doi.org/10.1080/09583157.2013.858100
- Randhawa N et al (2001) Management of root-knot nematode Meloidogyne incognita in tomato with organic amendments. Res Plant Dis 16:274–276
- Saikia ShK, Tiwari S, Pandey R (2013) Rhizospheric biological weapons for growth enhancement and Meloidogyne incognita management in Withania somnifera cv. Poshita. Biol Control 65:225–234
- Seenivasan N, David PMM, Vivekanandan P, Samiyappan R (2012) Biological control of rice root-knot nematode, Meloidogyne graminicola through mixture of Pseudomonas fluorescens strains. Biocontrol Sci Tecnol 22:611–632
- Siddiqui ZA, Mahmood I (1999) Role of bacteria in the management of plant parasitic nematodes: a review. Bioresour Technol 69:167–179
- Siddiqui IA, Shahid SS (2003) Suppression of root-knot disease by Pseudomonas fluorescens CHA0 in tomato: importance of bacterial secondary metabolite, 2,4-diacetylpholoroglucinol. Soil Biol Biochem 35:1615–1623
- Siddiqui ZA, Qureshi A, Akhtar MS (2009) Biocontrol of root-knot nematode Meloidogyne incognita by Pseudomonas and Bacillus isolates on Pisum sativum. Arch Phytopathol Plant Prot 42:1154–1164
- Silva RCFS, Almeida DG, Luna JM, Rufino RD, Santos VA, Sarubbo LA (2014) Applications of biosurfactants in the petroleum industry and the remediation of oil spills. Int J Mol Sci 15:12523–12542
- Son SH, Khan Z, Moon HS, Kim SG, Choi DR, Kim YH (2007) Nematicidal activity of a plant growth promoting rhizobacterium, Paenibacillus polymyxa. Russ J Nematol 15:95–100
- Staub T (1991) Fungicide resistance: practical experience with anti-resistance strategies and the role of integrated use. Annu Rev Phytopathol 29:421–442
- Stein T (2005) Bacillus subtilis antibiotics: structures, syntheses and specific functions. Mol Microbiol 56(4):845–857
- Sumeet, Mukerji KG (2000) Exploitation of protoplast fusion technology in improving biocontrol potential. In: Upadhyay RR, Mukerji KG, Chamola BP (eds) Biocontrol potential and its exploitation in sustainable agriculture, vol 1. Crop diseases, weeds, and nematodes. Kluwer Academic Plenum, New York, pp 39–48
- Terefe M, Tefera T, Sakhuja PK (2009) Effect of a formulation of Bacillus firmus on root-knot nematode Meloidogyne incognita infestation and the growth of tomato plants in the greenhouse and nursery. J Invertebr Pathol 100:94–99
- Tian H, Riggs RD, Crippen DL (2000) Control of soybean cyst nematode by chitinolytic bacteria with chitin substrate. J Nematol 32:370–376
- Timper P, Kone D, Yin J, Pingsheng JI, Brain B (2009) Evaluation of an antibiotic-producing isolate of Pseudomonas fluorescens for suppression of plant-parasitic nematodes. J Nematol 41:234–240 (PMid: 22736820 PMCid:PMC3380499)
- Tranier MS, Pognant-Gros J, De la Cruz Quiroz R, González CNA, Mateille T, Roussos S (2014) Commercial biological control agents targeted against plant-parasitic root-knot nematodes. Braz Arch Biol Technol 57(6):831–841
- Villareal RL (1980) Tomatoes in tropics. Western Press, New York, pp 174–175
- Walia RK, Sharma SB, Vats R (2000) Bacterial antagonists of phytonematodes. Biocontrol potential and its exploitation in sustainable agriculture. Springer, New York, pp 173–186
- Wei L-H, Xue Q-Y, Wei B-Q, Wang Y-M, Li S-M, Chen L-F, Guo J-H (2010) Screening of antagonistic bacterial strains against Meloidogyne incognita using protease activity. Biocontrol Sci Technol 20:739–750
- Yan F, Xu S, Guo J, Chen Q, Meng Q, Zheng X (2015) Biocontrol of postharvest Alternaria alternate decay of cherry tomatoes with rhamnolipids and possible mechanism of action. J Sci Food Agric 95:1469–1474
- Zhou D, Feng H, Schuelke T, De Santiago A, Zhang Q, Zhang J, Luo C, Wei L (2019) Rhizosphere microbiomes from root knot nematode non-infested plants suppress nematode infection. Microb Ecol 78(2):470–481. https://doi.org/10.1007/s00248-019-01319-5
Author Information
Plant Pathology and Nematology Section, Department of Botany, Aligarh Muslim University, Aligarh, India