Biogenic synthesis of selenium nanomaterial and its application as anti-Nematode booster in Solanum lycopersicum (Tomato)
Nikam Pradnya B., Salunkhe Jitendra D., Mohite Bhavana V., Chaudhari Rakesh S., Patil Satish V.
Research Articles | Published: 28 December, 2022
First Page: 1458
Last Page: 1464
Views: 3337
Keywords: Selenium nanoparticles, Nematicidal, Protease inhibition, Nano fertilizers, Meloidogyne
Abstract
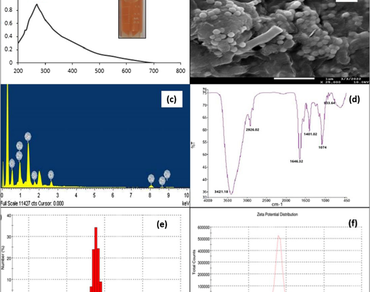
The biogenic selenium nanomaterial (SeNPs) are eco-friendly and reported as nano-fertilizers for alleviating the crops biotic and abiotic stresses. SeNPs were synthesized using a soil-friendly microbe, the Rhizobium sp., and used as a nematicidal booster in Solanum lycopersicum (Tomato) PUSA120. These SeNPs were characterized by SEM with EDX, and DLS determined the particle size. The UV Visible spectrum at 268 nm confirmed the synthesis of SeNPs. The aqueous concentrations of these nanomaterials, i.e., 5, 10, and 50 ppm, were tested as foliar spray treatment for up to 40 days. The plants were grown for 90 days and observed for the yield of crops artificially infected with Meloidogyne. Some treated and control plants were sacrificed after 40 days for morphological characterization and biomass analysis. The tested concentration of 10 ppm aqueous SeNPs spray showed a significant reduction in Meloidogyne infections, i.e., root galls, eggs in an ootheca, and increased biomass. After 90 days, it was observed that the 10 ppm treatment enhanced the tomato yield. The underlying mechanism was the induction of protease inhibitors in roots and leaves in the presence of nano selenium. A higher concentration, like 50 ppm, proved to be a growth inhibitor. Hence, SeNPs may be safe and a good economical agro material.
References
Athanassiou CG, Kavallieratos NG, Benelli G, Losic D, Usha Rani P, Desneux N (2018) Nanoparticles for pest control: current status and future perspectives. J Pest Sci 91(1):1–15. https://doi.org/10.1007/s10340-017-0898-0
Bardner R, Fletcher KE (1974) Insect infestations and their effects on the growth and yield of field crops: a review. Bull Entomol Res 64(1):141–160. https://doi.org/10.1017/S0007485300027061
Cavalu S, Kamel E, Laslo V, Fritea L, Costea T, Antoniac IV, Vasile E, Antoniac A, Semenescu A, Mohan A, Saceleleanu V (2017) Eco-friendly, facile and rapid way for synthesis of selenium nanoparticles. Rev Roum Chim 68:2963–2966
Cavalu S, Antoniac I, Fritea L, Mates IM, Milea C, Laslo V, Vicas S, Mohan A (2018) Surface modifications of the titanium mesh for cranioplasty using selenium nanoparticles coating. J Adhes Sci Technol 32(22):2509–2522. https://doi.org/10.1080/01694243.2018.1490067
Chhabria S, Desai K (2016) Selenium nanoparticles and their applications. Encyclop Nanosci Nanotechnol 20:1–32
El-Ramady H, Abdalla N, Taha HS, Alshaal T, El-Henawy A, Faizy SED, Shams MS, Youssef SM, Shalaby T, Bayoumi Y, Elhawat N (2016) Selenium and nano-selenium in plant nutrition. Environ Chem Lett 14(1):123–147. https://doi.org/10.1007/s10311-015-0535-1
Erlanger BF, Kokowsky N, Cohen W (1961) The preparation and properties of two new chromogenic substrates of trypsin. Arch Biochem Biophys 95(2):271–278. https://doi.org/10.1016/0003-9861(61)90145-X
Garza-García JJ, Hernández-Díaz JA, Zamudio-Ojeda A, León-Morales JM, Guerrero-Guzmán A, Sánchez-Chiprés DR, López-Velázquez JC, García-Morales S (2021) The role of selenium nanoparticles in agriculture and food technology. Biol Trace Elem Res 5:1–21. https://doi.org/10.1007/s12011-021-02847-3
Gomes CE, Barbosa AE, Macedo LL, Pitanga JC, Moura FT, Oliveira AS, Moura RM, Queiroz AF, Macedo FP, Andrade LB, Vidal MS (2005) Effect of trypsin inhibitor from Crotalaria pallida seeds on Callosobruchus maculatus (cowpea weevil) and Ceratitis capitata (fruit fly). Plant Physiol Biochem 43:1095–1102. https://doi.org/10.1016/j.plaphy.2005.11.004
Gupta M, Gupta S (2017) An overview of selenium uptake, metabolism, and toxicity in plants. Front Plant Sci 7:2074. https://doi.org/10.3389/fpls.2016.02074
Hartikainen H, Xue T, Piironen V (2000) Selenium as an anti-oxidant and pro-oxidant in ryegrass. Plant Soil 225:193–200. https://doi.org/10.1023/A:1026512921026
Hayat R, Ahmed I, Sheirdil RA (2012) An overview of plant growth promoting rhizobacteria (PGPR) for sustainable agriculture. Crop production for agricultural improvement. Springer, Berlin, pp 557–579. https://doi.org/10.1007/978-94-007-4116-4_22
Hernández-Hernández H, Quiterio-Gutiérrez T, Cadenas-Pliego G, Ortega-Ortiz H, Hernández-Fuentes AD, Cabrera de la Fuente M, Valdés-Reyna J, Juárez-Maldonado A (2019) Impact of selenium and copper nanoparticles on yield, antioxidant system, and fruit quality of tomato plants. Plants 8:355
Hosnedlova B, Kepinska M, Skalickova S, Hosnedlova B, Kepinska M, Skalickova S, Fernandez C, Ruttkay-Nedecky B, Peng Q, Baron M, Melcova M, Opatrilova R, Zidkova J, Bjørklund G (2018) Nano-selenium and its nanomedicine applications: a critical review. Int J Nanomed 13:2107. https://doi.org/10.2147/IJN.S157541
Husen A, Siddiqi KS (2014) Plants and microbes assisted selenium nanoparticles: characterization and application. J Nanotechnol 12(1):1–10. https://doi.org/10.1186/s12951-014-0028-6
Hussey RS, Barker KR (1973) A comparison of methods of collecting inocula of Meloidogyne spp., including a new technique. Plant Dis 57:1025–1028
Kondaparthi P, Flora SJS, Naqvi S (2019) Selenium nanoparticles: an insight on its Pro-oxidant and antioxidant properties. Front Nanosci Nanotechnol 6:1–5
Kumar CV, Karthick V, Inbakandan D, Kumar VG, Rene ER, Dhas TS, Ravi M, Sowmiya P, Das CA (2022) Effect of selenium nanoparticles induced toxicity on the marine diatom Chaetoceros gracilis. Process Saf Environ Prot 163:200–209
Moens M, Perry RN, Starr JL (2009) Meloidogyne species—a diverse group of novel and important plant parasites. Root-Knot Nematodes 20:483
Nandiyanto ABD, Oktiani R, Ragadhita R (2019) How to read and interpret FTIR spectroscope of organic material. Indones J Sci Technol 4:97–118
Nikam PB, Salunkhe N, Marathe V et al (2022) Isolation of selenium biotransforming microbes as new age bioinputs. Practical handbook on agricultural microbiology. Springer, New York, pp 243–247. https://doi.org/10.1007/978-1-0716-1724-3_31
Oerke EC (2005) Crop losses to pests. Agric Sci 144(1):31–43. https://doi.org/10.1017/S0021859605005708
Parveen A, Rao S (2015) Effect of nanosilver on seed germination and seedling growth in Pennisetumglaucum. J Clus Sci 26(3):693–701. https://doi.org/10.1007/s10876-014-0728-y
Purwaningsih S, Agustiyani D, Antonius S (2012) Diversity, activity, and effectiveness of Rhizobium bacteria as plant growth promoting rhizobacteria (PGPR) isolated from Dieng, central Java. Iran J Microbiol 13(1):130. https://doi.org/10.18502/ijm.v13i1.5504
Quinn CF, El-Mehdawi AF, Pilon-Smits EAH (2017) Ecology of selenium in plants. Selenium in plants, vol 11. Springer, Cham, pp 177–188. https://doi.org/10.1007/978-3-319-56249-0_11
Schiavon M, Pilon-Smits EA (2017) The fascinating facets of plant selenium accumulation–biochemistry, physiology, evolution and ecology. New Phytol 213(4):1582–1596. https://doi.org/10.1111/nph.14378
Sels J, Mathys J, De Coninck BM, Cammue BP, De Bolle MF (2008) Plant pathogenesis-related (PR) proteins: a focus on PR peptides. Plant Physiol Biochem 46(11):941–950
Sharon M, Choudhary AK, Kumar R (2010) Nanotechnology in agricultural diseases and food safety. J Phytol 2(4):78–82
Talavera M, Miranda L, Gómez-Mora JA, Vela MD, Verdejo-Lucas S (2019) Nematode management in the strawberry fields of southern Spain. Agronomy 9(5):252. https://doi.org/10.3390/agronomy9050252
Van der Putten WH, Cook R, Costa S, Davies KG, Fargette M, Freitas H, Hol WHG, Kerry BR, Maher N, Mateille T, Moens M (2006) Nematode interactions in nature: models for sustainable control of nematode pests of crop plants? Adv Agron 89:227–260. https://doi.org/10.1016/S0065-2113(05)89005-4
Vivek P, Prabhakaran S, Shankar SR (2013) Assessment of nutritional value in selected edible greens based on the chlorophyll content in leaves. J Plant Biol 3(5):45–49
Wadhwani SA, Shedbalkar UU, Singh R, Chopade BA (2016) Biogenic selenium nanoparticles: current status and future prospects. Appl Microbiol Biotechnol 100(6):2555–2566. https://doi.org/10.1007/s00253-016-7300-7
Author Information
School of Life Sciences, Kavayitri Bahinabai Chaudhari North Maharashtra University, Jalagoan, India