Comparative evaluation of antioxidant, antiglycation and α-glucosidase inhibitory potential of some indigenous medicinal Trigonella species
Research Articles | Published: 24 December, 2022
First Page: 1337
Last Page: 1346
Views: 4214
Keywords: Antiglycation, Antioxidant, India, n Trigonellan
Abstract
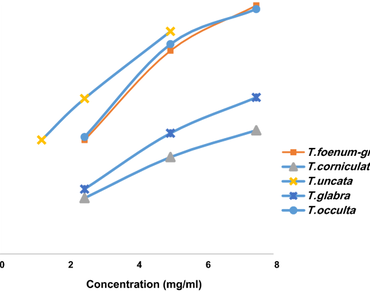
The aim of the present study was to comprehensively and comparatively investigate the antioxidant, antiglycation and α-glucosidase inhibitory potential of some indigenous medicinal Trigonella species. T. uncata with its higher phenolic and flavonoid content showed maximum inhibition of α-glucosidase activity as compared to other species. Methanolic seed extract of T. corniculata had maximum 2,2-Diphenyl-L-Picrylhydrazyl (DPPH) radical scavenging activity while that of T. glabra had maximum reducing power and total antioxidant activity. Aqueous seed extracts of all tested species exerted noticeable antiglycation potential with inhibition of fructosamine and β-amyloid aggregate formation (R = 0.950, p < 0.05) and reduction in carbonyl levels and increase in free thiol groups (R = 0.684, p < 0.05) well correlated. Results indicate that T. glabra, with its α-glycosidase inhibitory potential, higher antioxidant activity coupled with stronger protection of albumin against oxidation can be combined with T. foenum-graecum in polyherbal formulations for treating diabetic complications.
References
Abeysekera WKSM, Abayarathna UPTC, Premakumara GAS, Jayasooriya MCN, Abeysekera WPKM (2018) Anti-glycation and glycation reversing potential of fenugreek (Trigonella Foenum-Graecum) seed extract. Biomed J Sci Tech Res 3:1–5. https://doi.org/10.26717/BJSTR.2018.03.000875
Ardestani A, Yazdanparast R (2007) Antioxidant and free radical scavenging potential of Achillea santolina extracts. Food Chem 104:21–29
Baker JR, Zyzak DV, Thorpe SR, Baynes JW (1994) Chemistry of the fructosamine assay: D-glucosone is the product of oxidation of Amadori compounds. Clin Chem 40:1950–1955
Brand-Williams W, Cuvelier ME, Berset C (1995) Use of a free radical method to evaluate antioxidant activity. LWT Food Sci Technol 28:25–30
Chowdhury AA, Gawali NB, Bulani VD, Kothavade PS, Mestry SN, Deshpande PS, Juvekar AR (2018) In vitro antiglycating effect and in vivo neuroprotective activity of Trigonelline in d-galactose induced cognitive impairment. Pharmacol Rep 70:372–377. https://doi.org/10.1016/j.pharep.2017.09.006
Costa MC, Lima TFO, Arcaro CA, Inacio MD, Batista-Duharte A, Carlos IZ, Spolidorio LC, Assis RP, Brunetti IL, Baviera AM (2020) Trigonelline and curcumin alone, but not in combination, counteract oxidative stress and inflammation and increase glycation product detoxification in the liver and kidney of mice with high-fat diet-induced obesity. J Nutr Biochem 76:108303. https://doi.org/10.1016/j.jnutbio.2019.108303
Dendup T, Prachyawarakorn V, Pansanit A, Mahidol C, Ruchirawat S, Kittakoop P (2014) α-glucosidase inhibitory activities of isoflavanones, isoflavones, and pterocarpans from Mucuna pruriens. Planta Med 80:604–608. https://doi.org/10.1055/s-0034-1368427
Ellman GL (1995) Tissue sulfhydryl groups. Arch Biochem Biophys 82:70–77
Elosta A, Ghous T, Ahmed N (2012) Natural products as anti-glycation agents: possible therapeutic potential for diabetic complications. Curr Diabetes Rev 8:92–108
Fournet M, Bonté F, Desmoulière A (2018) Glycation damage: a possible hub for major pathophysiological disorders and aging. Aging Dis 9:880–900
Fuller S, Stephens JM (2015) Diosgenin, 4-hydroxyisoleucine, and fiber from fenugreek: mechanisms of actions and potential effects on metabolic syndrome. Adv Nutr 13:189–197
Ganeshpurkar A, Bhardwaj Y, Diwedi V (2013) In vitro α-amylase and α-glucosidase inhibitory potential of Trigonella foenum-graecum leaves extract. Int J Ayurveda Res 34:109. https://doi.org/10.4103/0974-8520.115446
Hamed AI (2007) Steroidal saponins from the seeds of Trigonella hamosa L. Nat Prod Commun 2:143–146
Hardman R, Fazil FRY (1972) Methods of screening the genus Trigonella for steroidal sapogenins. Planta Med 21:131–138
Jamuna KS, Ramesh CK, Srinivasa TR, Raghu KL (2011) Total antioxidant capacity in aqueous extracts of some common fruits. Int J of Pharm Sci Res 2:448–453
Kalita P, Barman TK, Pal TK, Kalita R (2013) Estimation of total flavonoids content (TFC) and antioxidant activities of methanolic whole plant extract of Biophytum sensitivum Linn. J Drug Delivery Thera 3:33–37
Kaur P, Kataria K, Singh B, Arora S (2018) Pharmacognostic profile of Trigonella corniculata L. seeds and effect of its aqueous extract on growth inhibition of cancer cells. Res J Pharm Technol 11:2022–2029
Khalil HE, Ibrahim HIM, Ahmed EA, Emeka PM, Alhaider IA (2022) Orientin, a bio-flavonoid from Trigonella hamosa L., regulates COX-2/PGE-2 in A549 cell lines via miR-26b and miR-146a. Pharmaceuticals 15:154. https://doi.org/10.3390/ph15020154
Khan J, Saeed MA, Touqeer S, Adnan S, Masood Z, Zaman M (2014) Antihyperglycemic, hypoglycemic and cytotoxic activity of Albizia lebbek and Trigonella corniculata. Research J Pharm and Tech 7:191–195
Kim KJ, Lee BW (2012) The roles of glycated albumin as intermediate glycation index and pathogenic protein. Diabetes Metab J 36:98–107
Klunk WE, Jacob RF, Mason RP (1999) Quantifying amyloid by Congo red spectral shift assay. Enzymol 309:285–305
Mandaville JP (2013) Flora of Eastern Saudi Arabia. Routledge, London, UK
McPherson JD, Shilton BH, Walton DJ (1988) Role of fructose in glycation and cross-linking of proteins. Biochem 27:1901–1907
Mittal K, Ingle P, Dangi R (2020) Trigonella glabra (Leguminosae): a new addition to Trigonella genetic resources in India. J Econ Taxon Bot 44:5–7
Naeem M, Aftab T, Khan M (2021) Fenugreek biology and applications. Springer Nature, Singapore
Nunthanawanich P, Sompong W, Sirikwanpong S et al (2016) Moringa oleifera aqueous leaf extract inhibits reducing monosaccharide-induced protein glycation and oxidation of bovine serum albumin. Springerplus 5:1098. https://doi.org/10.1186/s40064-016-2759-3
Oyaizu M (1986) Studies on products of browing reaction: Antioxidative activity of product of browing reaction preapared from glucosamine. Jap J Nurtion 44:307–315
Petropoulos GA (2002) Fenugreek The genus Trigonella. Taylor & Francis, London
Proença C, Freitas M, Ribeiro D, Oliveira EFT, Sousa JLC, Tomé SM, Ramos MJ, Silva AMS, Fernandes PA, Fernandes E (2017) α-Glucosidase inhibition by flavonoids: an in vitro and in silico structure-activity relationship study. J Enzyme Inhib Med Chem 32:1216–1228. https://doi.org/10.1080/14756366.2017.1368503
Qari SH, Fahmy NM (2017) Evaluation of some biological activities of Trigonella hamosa aerial parts. J Pharmacogn Phytother 9:165–172
Quattrocchi U (2016) CRC World Dictionary of Medicinal and Poisonous Plants: Common Names, Scientific Names, Eponyms, Synonyms, and Etymology (5 volume set). CRC Press, Taylor & Francis, Boca Raton
Riyaphan J, Jhong CH, Tsai MJ, Lee DN, Leong MK, Weng CF (2017) Potent natural inhibitors of alpha-glucosidase and alpha-amylase against hyperglycemiain in vitro and in vivo. Prepint 1–20
Sadowska-Bartosz I, Bartosz G (2015) Prevention of protein glycation by natural compounds. Molecules 20:3309–3334
Salah-Eldin A, Mahalel UA, Hamed A (2007) Protective role of Trigonella hamosa saponins against diabetic perturbations and complications in rats. Nat pro Commun 2:811–816
Salman MT, Qadeer F (2012) Pharmacological actions and therapeutic potential of Trigonella foenum-graecum L.. In: Naeem M, Aftab T, Khan M (eds) Fenugreek biology and applications. Springer Nature, Singapore, pp 504–523
Semalty M, Semalty A, Joshi GP, Rawat MSM (2009) Comparison of in vitro activity of Trigonella foenum-graecum and T. corniculata seeds. Res J Phytochem 3:63–67
Shahat AA, Ibrahim AY, Alsaid MS (2015) Antioxidant capacity and polyphenolic content of seven Saudi Arabian medicinal herbs traditionally used in Saudi Arabia. Indian J Tradit Knowl 2:28–35
Shawky E, Sobhy A, Ghareeb DA, Eldin SM, Selim DA (2022) Comparative metabolomics analysis of bioactive constituents of the leaves of different Trigonella species: correlation study to α-amylase and α-glycosidase inhibitory effects. Ind Crops Prod 182:1–13. https://doi.org/10.1016/j.indcrop.2022.114947
Singleton VL, Orthofer R, Lamuela-Raventós RM (1999) Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Meth Enzymol 299:152–178
Song Q, Liu J, Dong L, Wang X, Zhang X (2021) Novel advances in inhibiting advanced glycation end product formation using natural compounds. Biomed Pharmacother 140:111750. https://doi.org/10.1016/j.biopha.2021.111750
Tabassum H, Ahmad IZ (2021) Trigonella foenum-graecum and its bioactive compounds having potential antidiabetic activity. In: Naeem M, Aftab T, Khan M (eds) Fenugreek biology and applications. Springer Nature, Singapore, pp 447–480
Telagari M, Hullatti K (2015) In-vitro α-amylase and α-glucosidase inhibitory activity of Adiantum caudatum Linn. and Celosia argentea Linn. extracts and fractions. Indian J of Pharmacol 47:425–429
Uchida KM, Kanematsu K, Sakai T (1998) Protein-bound macrolein: potential markers for oxidative stress (covalent modification of protein/antibody/atherosclerosis). Proc Natl Acad Sci 95:4882–4887
Younus H, Anwar S (2016) Prevention of non-enzymatic glycosylation (glycation): implication in the treatment of diabetic complication. Int J Health Sci (qassim) 10:261–277
Zhang QW, Lin LG, Ye WC (2018) Techniques for extraction and isolation of natural products: a comprehensive review. Chin Med 13:20. https://doi.org/10.1186/s13020-018-0177-x
Author Information
Department of Cell and Molecular Biology, Bharati Vidyapeeth (Deemed to be) University, Rajiv Gandhi Institute of IT and Biotechnology, Pune, India