Differential in vitro morphology exhibited by pigeonpea seedlings in response to plant growth regulators
Research Articles | Published: 26 February, 2022
First Page: 656
Last Page: 662
Views: 4016
Keywords: Pigeonpea, Apical dominance, In vitro, Growth regulators, Morphology
Abstract
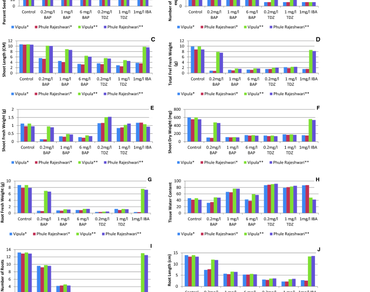
The effect of benzyl aminopurine (BAP), thidiazuron (TDZ), and indole butyric acid (IBA) on seedling morphology in two pigeonpea varieties, viz ‘Vipula’ and ‘Phule Rajeshwari’ were studied. Primary shoot growth suppression occurs at higher growth regulator concentration, leading to the outgrowth of axillary buds. Prevention of axillary bud initiation in auxin supplemented medium emphasizes the role of auxin in apical dominance. The adventitious shoot buds, bordered by a spiral of meristematic tissue, were formed at the pre-existing axillary bud in a cytokinin supplemented medium. The highest multiple shoot formation (5.7 per explants) was observed on 0.2 mg/l BAP supplemented MS medium. Plant growth regulators induced many shoot and root morphology variations in both cultivars. The numbers of roots per seedling varied from 13.2 to 1. A higher shoot abnormality was recorded in seedlings grown in TDZ supplemented medium. The current study highlighted how plant growth regulators influence the morphological response of in vitro regenerated plants.
References
Aremu AO, Stirk WA, Masondo NA, Plackova L, Novak O, Pencik A, Zatloukalb M, Nislerb J, SpIchalb L, Doleza K, Finniea J, Finnie JF (2015) Dissecting the role of two cytokinin analogues (INCYDE and PI-55) on in vitro organogenesis, phytohormone accumulation, phytochemical content, and antioxidant activity. Plant Sci 238:81–94. https://doi.org/10.1016/j.plantsci.2015.05.018
Chandra A, Gupta V, Burma P, Pental D (2003) Patterns of morphogenesis from cotyledon explants of pigeon pea. In Vitro Cell Dev Biol Plant 39:514–519. https://doi.org/10.1079/IVP2003437
Food and Agriculture Organization of the United Nations (2022) FAOSTAT Database Rome Italy. FAO Available at: http://faostat.fao.org/database
Franklin G, Jeyachandran R, Melchias G, Ignacimuthu S (1998) Multiple shoot induction and regeneration of pigeonpea (Cajanuscajan (L.) Millsp.) cv. Vamban: from apical and axillary meristem. Curr Sci 74:936–937
Franklin G, Jeyachandran R, Ignacimuthu S (2000) Factors affecting regeneration of pigeon pea (Cajanuscajan L. Millsp) from mature embryonal axes. Plant Growth Regul 30:31–36. https://doi.org/10.1023/A:1006394402210
Ganguly S, Ghosh G, Purohit A, Chaudhuri RK, Chakraborti D (2018) Development of transgenic pigeonpea using high throughput plumular meristem transformation method. Plant Cell Tissue Org Cult 135:73–83. https://doi.org/10.1007/s11240-018-1444-3
George L, Eapen S (1994) Organogenesis and embryogenesis from diverse explants in pigeon pea (Cajanuscajan L.). Plant Cell Rep 13:417–420. https://doi.org/10.1007/BF00234150
Ghosh G, Purohit A, Ganguly S, Chaudhuri RK, Chakraborti D (2014) In vitro shoot grafting on rootstock: an effective tool for Agrobacterium-mediated transformation of pigeonpea (Cajanus cajan (L.) Millsp.). Plant Biotechnol 31(4):301–308. https://doi.org/10.5511/plantbiotechnology.14.0805a
Krishna G, Reddy PS, Ramteke PW, Rambabu P, Tawar KB, Bhattacharya P (2011) Agrobacterium-mediated genetic transformation of pigeon pea [Cajanus cajan (L.) Mill sp.] for resistance to legume pod borer Helicoverpa armigera. J Crop Sci Biotech 14 (3): 197–204. https://doi.org/10.1007/s12298-011-0079-1
Mohan ML, Krishnamurthy KV (1998) Plant regeneration in pigeon pea (Cajanus cajan (L.) Mill sp.) by organogenesis. Plant Cell Rep 17:705–710. https://doi.org/10.1007/s002990050469
Muller D, Waldie T, Miyawaki K, To JPC, Melnyk CW, Kieber JJ, Kakimoto T, Leyser O (2015) Cytokinin is required for escape but not release from auxin mediated apical dominance. Plant J 82:874–886. https://doi.org/10.1111/tpj.12862
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassay with tobacco tissue cultures. Physiol Plant 15:473–497. https://doi.org/10.1111/j.1399-3054.1962.tb08052.x
Pawar BD, Jadhav AS, Kale AA, Chimote VP, Pawar SV (2012) Thidiazuron improves in vitro multiple shoot induction in chickpea (Cicer arietinum L.) cotyledon with embryonic axis. J Food Legumes 25(1): 9–13.
Pawar BD, Jadhav AS, Pawar SV, Chimote VP, Kale AA (2014) An efficient regeneration system for pigeonpea (Cajanus cajan L.) J Crop Improvement 28(6):825–833
Sarkar S, Roy S, Ghosh SK, Basu A (2019) Application of lateral branching to overcome the recalcitrance of in vitro regeneration of Agrobacterium infected pigeonpea (Cajanus cajan L.). Plant Cell Tiss Organ Cult 137:23–32. https://doi.org/10.1007/s11240-018-01547-6
Sarkar S, Roy S, Ghosh SK (2021) Development of marker-free transgenic pigeon pea (Cajanus cajan) expressing a pod borer insecticidal protein. Sci Rep 11:10543. https://doi.org/10.1038/s41598-021-90050-8
Singh H, Dara BL (1971) Influence of presoaking of seeds with gibberellin and auxins on growth and yield attributes of wheat (Triticum aestivum L.) under high salinity, sodium adsorption ratio and boron levels. Indian J Agric Sci 41:998–1003
Singh ND, Sahoo L, Sarin NB, Jaiwal PK (2003) The effect of TDZ on organogenesis and somatic embryogenesis in pigeonpea (CajanuscajanL. Millsp). Plant Sci 164:341–347. https://doi.org/10.1016/S0168-9452(02)00418-1
Tanaka M, Takei K, Kojima M, Sakakibara H, Mori H (2006) Auxin controls local cytokinin biosynthesis in the nodal stem in apical dominance. Plant J 45:1028–1036. https://doi.org/10.1111/j.1365-313X.2006.02656.x
Thu TT, Dewaele E, Trung LQ, Claeys M, Jacobs M, Angenon G (2007) Increasing lysine levels in pigeon pea (Cajanuscajan (L.) Millsp.) seeds through genetic engineering. Plant Cell Tiss Org Cult 91:135–143. https://doi.org/10.1007/s11240-007-9227-2
Author Information
State Level Biotechnology Centre, Mahatma Phule Agricultural University, Rahuri, India