Edible microalgae: potential candidate for developing edible vaccines
Jiji Merin Grace, Ninan Merin Ann, Thomas V. P., Thomas Binoy T.
Review Articles | Published: 27 April, 2023
First Page: 788
Last Page: 793
Views: 3731
Keywords: Chloroplast, Edible vaccine, Immunostimulants, Microalgae
Abstract
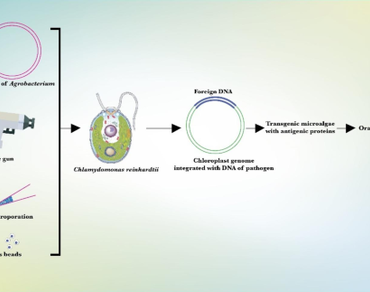
Infectious diseases are always a threat to all living beings. Today, in this world pathogens have no difficulty reaching anywhere. Every year new and deadly diseases are born and most of them are caused by viruses. Vaccines can provide lifelong immunity against infectious diseases, but the production cost of vaccines is unaffordable for a layman and traditional vaccines have certain limitations with storage and delivery. However, edible vaccines have shifted this paradigm and have received acceptance all over the world, especially in developing countries. Microalgae are one of the potential candidates for developing edible vaccines. Modifying microalgae as edible vaccines are gaining worldwide attention, especially in the world of science. Microalgae can augment the immune system as they are a promising source for antigen carriers and many of them are regarded as safe to eat. Moreover, they are a pantry of proteins, vitamins, minerals, and other secondary metabolites like alkaloids, phenols, and terpenes. In addition, being resistant to animal pathogens they are less sophisticated for genetic modification. This review analyses the potential scope of microalgae as an edible vaccine source.
References
Andre F, Booy R, Bock H et al (2008) Vaccination greatly reduces disease, disability, death and inequity worldwide. Bull World Heal Organ 86:81–160
Bai LL, Yin WB, Chen YH et al (2013) A New Strategy to produce a defensin: stable production of mutated NP-1 in Nitrate reductase-deficient Chlorella ellipsoidea. PLoS ONE 8:1–9. https://doi.org/10.1371/journal.pone.0054966
Barahimipour R, Neupert J, Bock R (2016) Efficient expression of nuclear transgenes in the green alga Chlamydomonas: synthesis of an HIV antigen and development of a new selectable marker. Plant Mol Biol 90:403–418. https://doi.org/10.1007/s11103-015-0425-8
Barney J (2017) Antibiotics found to weaken immune response to disease. https://news.virginia.edu/content/antibiotics-found-weaken-immune-response-disease. Accessed 7 Feb 2021
Bhatia S, Dahiya R (2015) Edible vaccines. Modern applications of Plant Biotechnology in Pharmaceutical Sciences. Elsevier Inc., pp 333–343
Bloom DE, Cadarette D (2019) Infectious disease threats in the twenty-first century: strengthening the global response. Front Immunol 10. https://doi.org/10.3389/fimmu.2019.00549
Borowitzka MA (2013) High-value products from microalgae-their development and commercialisation. J Appl Phycol 25:743–756. https://doi.org/10.1007/s10811-013-9983-9
Charoonnart P, Purton S, Saksmerprome V (2018) Applications of microalgal biotechnology for disease control in aquaculture. Biology (Basel) 7:1–14. https://doi.org/10.3390/biology7020024
Chebolu S, Daniell H (2010) Chloroplast-derived vaccine antigens and biopharmaceuticals: expression, folding, assembly and functionality. Curr Top Microbiol Immunol 332:33–54. https://doi.org/10.1007/978-3-540-70868-1_3
Costa JAV, Moreira JB, Fanka LS et al (2020) Microalgal biotechnology applied in biomedicine. In: Ozcan K (ed) Handbook of Algal Science, Technology and Medicine. Mathew Deans, pp 429–439.
Criscuolo E, Caputo V, Diotti RA et al (2019) Alternative methods of vaccine delivery: an overview of edible and intradermal vaccines. J Immunol Res 2019:1–13. https://doi.org/10.1155/2019/8303648
Daniell H, Chebolu S, Kumar S et al (2005) Chloroplast-derived vaccine antigens and other therapeutic proteins. Vaccine 23:1779–1783. https://doi.org/10.1016/j.vaccine.2004.11.004
Dehghani J, Adibkia K, Movafeghi A et al (2018) Stable transformation of Spirulina (Arthrospira) platensis: a promising microalga for production of edible vaccines. Appl Microbiol Biotechnol 102:9267–9278. https://doi.org/10.1007/s00253-018-9296-7
Demurtas OC, Massa S, Ferrante P et al (2013) A Chlamydomonas-Derived Human Papillomavirus 16 E7 vaccine induces specific Tumor Protection. PLoS ONE 8:e61473. https://doi.org/10.1371/journal.pone.0061473
Diaz CJ, Douglas KJ, Kang K et al (2023) Developing algae as a sustainable food source. Front Nutr 9:1–21. https://doi.org/10.3389/fnut.2022.1029841
Dreesen IAJ, Hamri GC, El, Fussenegger M (2010) Heat-stable oral alga-based vaccine protects mice from Staphylococcus aureus infection. J Biotechnol 145:273–280. https://doi.org/10.1016/j.jbiotec.2009.12.006
Dyo YM, Purton S (2018) The algal chloroplast as a synthetic biology platform for production of therapeutic proteins. Microbiology 164:113–121. https://doi.org/10.1099/mic.0.000599
Elaya Perumal U, Sundararaj R (2020) Algae: a potential source to prevent and cure the novel coronavirus – a review. Int J Emerg Technol 11:479–483.
Eriksen NT (2008) Production of phycocyanin - A pigment with applications in biology, biotechnology, foods and medicine. Appl Microbiol Biotechnol 80:1–14. https://doi.org/10.1007/s00253-008-1542-y
Food and Agricultural Organization of the United Nations (2021) Global seaweeds and microalgae production, 1950–2019
Franco EL, de Sanjosé S, Broker TR et al (2012) Human papillomavirus and cancer prevention: gaps in knowledge and prospects for research, policy, and advocacy. Vaccine 30:F175–F182. https://doi.org/10.1016/j.vaccine.2012.06.092
Gangl D, Zedler JAZ, Rajakumar PD et al (2015) Biotechnological exploitation of microalgae. J Exp Bot 66:6975–6990. https://doi.org/10.1093/jxb/erv426
Gartland KMA, Bruschi F, Dundar M et al (2013) Progress towards the “Golden Age” of biotechnology. Curr Opin Biotechnol 24:S6–S13. https://doi.org/10.1016/j.copbio.2013.05.011
Geng D, Wang Y, Wang P et al (2003) Stable expression of hepatitis B surface antigen gene in Dunaliella salina (Chlorophyta). J Appl Phycol 15:451–456. https://doi.org/10.1023/B:JAPH.0000004298.89183.e5
Gregory JA, Li F, Tomosada LM et al (2012) Algae-produced pfs25 elicits antibodies that inhibit malaria transmission. PLoS ONE 7:1–10. https://doi.org/10.1371/journal.pone.0037179
Gregory JA, Topol AB, Doerner DZ, Mayfield S (2013) Alga-produced cholera toxin-Pfs25 fusion proteins as oral vaccines. Appl Environ Microbiol 79:3917–3925. https://doi.org/10.1128/AEM.00714-13
Gunasekaran B, Gothandam KM (2020) A review on edible vaccines and their prospects. Brazilian J Med Biol Res 53:1–10. https://doi.org/10.1590/1414-431x20198749
Gutiérrez CL, Gimpel J, Escobar C et al (2012) Chloroplast genetic tool for the green microalgae Haematococcus pluvialis (chlorophyceae, volvocales). J Phycol 48:976–983. https://doi.org/10.1111/j.1529-8817.2012.01178.x
Habibi-Pirkoohi M, Mohkami A (2015) Recombinant vaccine production in green plants: state of art. J Cell Mol Res 7:59–67. https://doi.org/10.22067/jcmr.v7i1.45074
Hallmann A (2015) Algae Biotechnology – Green cell-factories on the rise. Curr Biotechnol 4:389–415. https://doi.org/10.2174/2211550105666151107001338
He DM, Qian KX, Shen GF et al (2007) Recombination and expression of classical swine fever virus (CSFV) structural protein E2 gene in Chlamydomonas reinhardtii chroloplasts. Colloids Surf B Biointerfaces 55:26–30. https://doi.org/10.1016/j.colsurfb.2006.10.042
Horizon (2020) Algae for fish vaccine. https://www.enabling-project.com/news-1/2020/2/11/algae-for-fish-vaccine. Accessed 5 Feb 2021
Jan N, Shafi F, Hameed O, bin et al (2016) An overview on edible vaccines and immunization. Austin J Nutr Food Sci 4:1078.
Khan MI, Shin JH, Kim JD (2018) The promising future of microalgae: current status, challenges, and optimization of a sustainable and renewable industry for biofuels, feed, and other products. Microb Cell Fact 17:1–21. https://doi.org/10.1186/s12934-018-0879-x
Kiataramgul A, Maneenin S, Purton S et al (2020) An oral delivery system for controlling white spot syndrome virus infection in shrimp using transgenic microalgae. Aquaculture 521:735022. https://doi.org/10.1016/j.aquaculture.2020.735022
Kothari R, Pandey A, Ahmad S et al (2017) Microalgal cultivation for value-added products: a critical enviro-economical assessment. 3 Biotech 7. https://doi.org/10.1007/s13205-017-0812-8
Koyande AK, Chew KW, Rambabu K et al (2019) Microalgae: a potential alternative to health supplementation for humans. Food Sci Hum Wellness 8:16–24. https://doi.org/10.1016/j.fshw.2019.03.001
Kurup VM, Thomas J (2020) Edible vaccines: promises and challenges. Mol Biotechnol 62:79–90. https://doi.org/10.1007/s12033-019-00222-1
Kusmayadi A, Leong YK, Yen HW et al (2021) Microalgae as sustainable food and feed sources for animals and humans – biotechnological and environmental aspects. Chemosphere 271:129800. https://doi.org/10.1016/j.chemosphere.2021.129800
Larosa V, Remacle C (2013) Transformation of the mitochondrial genome. Int J Dev Biol 57:659–665. https://doi.org/10.1387/ijdb.130230cr
León-Bañares R, González-Ballester D, Galván A, Fernández E (2004) Transgenic microalgae as green cell-factories. Trends Biotechnol 22:45–52. https://doi.org/10.1016/j.tibtech.2003.11.003
Liang ZC, Liang MH, Jiang JG (2020) Transgenic microalgae as bioreactors. Crit Rev Food Sci Nutr 60:3195–3213. https://doi.org/10.1080/10408398.2019.1680525
Madhav N, Oppenheim B, Gallivan M et al (2018) pandemics:Risks,Impact and Mitigation. In: Jamison DT, Gelband H, Horton S,. (eds) Disease Control Priorities, 3rd edition: improving health and reducing poverty, 3rd edn. pp 315–345.
Mayfield SP, Franklin SE, Lerner RA (2003) Expression and assembly of a fully active antibody in algae. Proc Natl Acad Sci U S A 100:438–442. https://doi.org/10.1073/pnas.0237108100
Mobin S, Alam F (2017) Some promising Microalgal species for commercial applications: a review. Energy Procedia 110:510–517. https://doi.org/10.1016/j.egypro.2017.03.177
Norero D (2020) Italy and Israel bet on GM microalgae to develop edible COVID vaccine. https://allianceforscience.cornell.edu/blog/2020/06/italy-and-israel-bet-on-gm-microalgae-to-develop-edible-covid-vaccine/. Accessed 5 Feb 2021
Okuzumi J, Nishino H, Murakoshi M et al (1990) Inhibitory effects of fucoxanthin, a natural carotenoid, on N-myc expression and cell cycle progression in human malignant tumor cells. Cancer Lett 55:75–81. https://doi.org/10.1016/0304-3835(90)90068-9
Potvin G, Zhang Z (2010) Strategies for high-level recombinant protein expression in transgenic microalgae: a review. Biotechnol Adv 28:910–918. https://doi.org/10.1016/j.biotechadv.2010.08.006
Ramos-Vega A, Rosales-Mendoza S, Bañuelos-Hernández B, Angulo C (2018) Prospects on the use of Schizochytrium sp. to develop oral vaccines.Front Microbiol 9. https://doi.org/10.3389/fmicb.2018.02506
Rasala BA, Muto M, Lee PA et al (2010) Production of therapeutic proteins in algae, analysis of expression of seven human proteins in the chloroplast of Chlamydomonas reinhardtii. Plant Biotechnol J 8:719–733. https://doi.org/10.1111/j.1467-7652.2010.00503.x
Reed SG, Orr MT, Fox CB (2013) Key roles of adjuvants in modern vaccines. Nat Med 19:1597–1608. https://doi.org/10.1038/nm.3409
Robertson S (2021) New platform using green algae tests for SARSCoV2 virus. https://www.news-medical.net/news/20210201/New-platform-using-green-algae-tests-for-SARS-CoV-2-virus.aspx. Accessed 8 Feb 2021
Rosales-Mendoza S (2016) Algae-based biopharmaceuticals. Algae-Based Biopharm 1–166. https://doi.org/10.1007/978-3-319-32232-2
Rosales-Mendoza S, García-Silva I, González-Ortega O et al (2020) The potential of Algal Biotechnology to produce antiviral compounds and biopharmaceuticals. Molecules 25:1–25. https://doi.org/10.3390/molecules25184049
Sahoo A, Mandal AK, Dwivedi K, Kumar V (2020) A cross talk between the immunization and edible vaccine: current challenges and future prospects. Life Sci 261:118843. https://doi.org/10.1016/j.lfs.2020.118343
Saveria T, Parthiban C, Seilie AM et al (2022) Needle-free, spirulina-produced Plasmodium falciparum circumsporozoite vaccination provides sterile protection against pre-erythrocytic malaria in mice. npj Vaccines 7:1–11. https://doi.org/10.1038/s41541-022-00534-5
Saxena J, Rawat S (2013) Edible vaccines. In: Ravi I, Baunthial M, Saxena J (eds) Advances in Biotechnology. Springer New Delhi, pp 207–226
Shiraishi H, Tabuse Y (2013) The AplI restriction-modification system in an edible cyanobacterium, arthrospira (spirulina) platensis NIES-39, recognizes the nucleotide sequence 5′-CTGCAG-3′. Biosci Biotechnol Biochem 77:782–788. https://doi.org/10.1271/bbb.120919
Specht EA, Mayfield SP (2014) Algae-based oral recombinant vaccines. Front Microbiol 5:1–7. https://doi.org/10.3389/fmicb.2014.00060
Streatfield SJ, Jilka JM, Hood EE et al (2001) Plant-based vaccines: unique advantages. Vaccine 19:2742–2748. https://doi.org/10.1016/S0264-410X(00)00512-0
Sun M, Qian K, Su N et al (2003) Foot-and-mouth disease virus VP1 protein fused with cholera toxin B subunit expressed in Chlamydomonas reinhardtii chloroplast. Biotechnol Lett 25:1087–1092. https://doi.org/10.1023/A:1024140114505
Takeyama N, Kiyono H, Yuki Y (2015) Plant-based vaccines for animals and humans: recent advances in technology and clinical trials. Ther Adv Vaccines 3:139–154. https://doi.org/10.1177/2051013615613272
Taunt HN, Stoffels L, Purton S (2018) Green biologics: the algal chloroplast as a platform for making biopharmaceuticals. Bioengineered 9:48–54. https://doi.org/10.1080/21655979.2017.1377867
Vetter V, Denizer G, Friedland LR et al (2018) Understanding modern-day vaccines: what you need to know. Ann Med 50:110–120. https://doi.org/10.1080/07853890.2017.1407035
Waheed S, Bayas A, Hindi F et al (2021) Neurological complications of COVID-19: Guillain-Barre Syndrome following Pfizer COVID- 19 vaccine. Cureus 13:2–5. https://doi.org/10.7759/cureus.13426
Author Information
Phycotechnology laboratory, Post Graduate and Research Department of Botany, Pathanamthitta, India