Enhancing conservation and genetic resource management through cross-transferability exploration and optimization of DNA extraction protocol for Buchanania cochinchinensis (Lour.) M. R. Almeida
*Article not assigned to an issue yet
Dahayat Ankur, Mohammad Naseer, Yadav Raj Singh, Agrahari Harshita, Pardhi Yogesh, Shirin Fatima, Miskini Bilal
Research Articles | Published: 29 January, 2025
First Page: 0
Last Page: 0
Views: 1733
Keywords: DNA, Microsatellite, n Buchanania Lanzann , Anacardiaceae, Marker development
Abstract
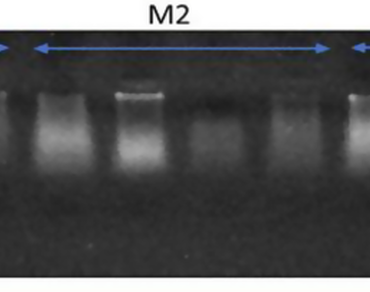
Buchanania cochinchinensis (Lour.) Almeda, a socioeconomically important tree in the Anacardiaceae family, is valued for its versatile use, afforestation potential and medicinal value. Despite its socioeconomic significance, molecular studies on this species using microsatellite markers are lacking. The present study optimized a high-throughput DNA extraction protocol for fresh and stored leaf samples, addressing the challenges posed by secondary metabolites in such forest trees. The isolated DNA was checked via UV Spectrophotometry, which revealed A260/A280 ratios ranging from 1.65 to 1.83, indicating the suitability of the isolated DNA for polymerase chain reaction (PCR) amplification. Owing to the unavailability of specific microsatellite primers for B. cochinchinensis, a cross-species transferability approach was employed using microsatellite markers from other species, i.e., Cotinus coggygria and Mangifera indica of the Anacardiaceae family. Among the fourteen SSR primers screened, four exhibited cross-species transferability and were further characterized. Marker characteristics such as heterozygosity (mean H: 0.42), polymorphic information content (mean PIC: 0.33), effective multiplex ratio (mean: 1.0), discrimination power (mean DP: 0.71), and resolving power (mean RP: 0.92) were evaluated. The gene diversity (mean = 0.38) and Shannon information index (mean = 0.56) were also estimated. These findings indicate that the cross-transferred microsatellite markers are effective for genetic studies in B. cochinchinensis. These markers can be utilized for further molecular characterization and genetic diversity assessment of this economically important species.
References
Agrawal T, Quraishi A (2023) Assessing the genetic diversity of Buchanania Lanzan Spreng. (Chironji) using inter-simple sequence repeat markers. Genet Resour Crop Evol 13:1–3. https://doi.org/10.1007/s10722-023-01812-4
Aiello D, Ferradini N, Torelli L, Volpi C, Lambalk J, Russi L et al (2020) Evaluation of cross-species transferability of SSR markers in Foeniculum vulgare. Plants 9:2–12. https://doi.org/10.3390/plants9020175
Ambreen H, Kumar S, Variath MT, Joshi G, Bali S, Agarwal M, Kumar A, Jagannath A, Goel S (2015) Development of genomic microsatellite markers in Carthamus tinctorius L.(safflower) using next generation sequencing and assessment of their cross-species transferability and utility for diversity analysis. PLoS ONE 10(8):e0135443. https://doi.org/10.1371/journal.pone.0135443
Amiryousefi A, Hyvonen J, Poczai P (2018) iMEC: online marker efficiency calculator. Appl Plant Sci 6:1–4. https://doi.org/10.1002/aps3.1159
Anand RK, Rao AP, Khare N, Singh SK (2021) Prospects and Potential of Chironji (Buchanania lanzan Spreng.) based Agroforestry for Livelihood and Environmental security in Central India. Agroforestry prospective, strategies and future aspects pp. 198–202
Anuradha HJ, Vijayan K, Nair CV, Manjula A (2013) A novel and efficient protocol for the isolation of genomic DNA from mulberry (Morus L.). Emir. J Food Agric 25:124–131
Barstow M (2024) Buchanania Lanzan. IUCN Red List Threatened Species 2024(eT32614A218855619). https://doi.org/10.2305/IUCN.UK.2024-1.RLTS.T32614A218855619.en. Accessed on 03 January 2025
Botstein D, White RL, Skolnick M, Davis RW (1980) Construction of a genetic linkage map in man using restriction fragment length polymorphisms. Am J Hum Genet 32:314–331
Chiang YC, Tsai CM, Chen YK, Lee SR, Chen CH, Lin YS, Tsai CC (2012) Development and characterization of 20 new polymorphic microsatellite markers from Mangifera indica (Anacardiaceae). Am J Bot 99(3):e117–119. https://doi.org/10.3732/ajb.1100443
Dabral A, Shamoon A, Meena RK, Kant R, Pandey S, Ginwal HS, Bhandari MS (2021) Genome skimming-based simple sequence repeat (SSR) marker discovery and characterization in Grevillea robusta. Physiol Mol Biology Plants 27:1623–1638. https://doi.org/10.1007/s12298-021-01035-w
Dahayat A, Singh N, Pardhi Y, Mohammad M (2017) Molecular marker and its scope in forestry. Van Sangyan 4:1–7
Deshmukh VP, Thakare PV, Chaudhari US, Gawande PA (2007) A simple method for isolation of genomic DNA from fresh and dry leaves of Terminalia Arjuna (Roxb.) Wight and Arnot. Electron J Biotechnol 10:468–472
Doyle JJ, Doyle JL (1987) A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochemical Bull 19:11–15
Ekue MR, Gailing O, Finkeldey R (2009) Transferability of simple sequence repeat (SSR) markers developed in Litchi chinensis to Blighia sapida (Sapindaceae). Plant Mol Biol Rep 27:570–574. https://doi.org/10.1007/s11105-009-0115-2
Elhaj AA, Gamra LH (2021) DNA extraction protocol for tomato and Arabidopsis plants using Edwards’ buffer. J Genet Genom Plant Breed 5:47–51
Fan L, Zhang MY, Liu QZ, Li LT, Song Y, Wang LF, Zhang SL, Wu J (2013) Transferability of newly developed pear SSR markers to other Rosaceae species. Plant Mol Biology Report 31:1271–1282. https://doi.org/10.1007/s11105-013-0586-z
Ghate U, Nydu P, Verma H, Ashraf S (2015) Climate Change & NTFP-livelihood implications for the tribal. CCD report supported by Indo Global Social Service Society, New Delhi
Ginwal HS, Mittal N (2010) An efficient genomic DNA isolation protocol for RAPD and SSR analysis in Acorus calamus L. Indian J Biotechnol 9:213–216
Jatav P, Tenguria RK (2022) Determination of total alkaloids and tannins contents in leaves of Buchanania Lanzan. J Coast Life Med 10:693–700
Jia BG, Lin Q, Feng YZ, Hu XY, Tan XF, Shao FG, Zhang L (2015) Development and cross-species transferability of unigene-derived microsatellite markers in an edible oil woody plant, Camellia Oleifera (Theaceae). Genet Mol Res 14(2):6906–6916. https://doi.org/10.4238/2015.June.18.33
Khanuja SP, Shasany AK, Darokar MP, Kumar S (1999) Rapid isolation of DNA from dry and fresh samples of plants producing large amounts of secondary metabolites and essential oils. Plant Mol Biol Rep 17:1–7. https://doi.org/10.1023/A:1007528101452
Kirtikar KR, Basu BD (2005) Text book of Indian medicinal plants. Dehradun, India: International Book Distributors p. 2:993–994
Kosman E (2003) Nei’s gene diversity and the index of average differences are identical measures of diversity within populations. Plant Pathol 52:533–535. https://doi.org/10.1046/j.1365-3059.2003.00923.x
Malakar A, Sahoo H, Sinha A, Kumar A (2023) Necessity of genetic diversity study and conservation practices in chironji (Buchanania cochinchinensis (lour.) M.R. Almeida). Environ Conserv J 24:253–260. https://doi.org/10.36953/ECJ.12802358
Malik SK, Choudery R, Panwar NS, Dhariwal OP, Ravish R, Kumkar S (2012) Genetic resources of Chironji (Buchanania Lanzan Spreng.): a socio-economic important tree species of central Indian population. Genet Resour Crop Evol 59:615–623. https://doi.org/10.1007/s10722-012-9801-2
Mehta SK, Jaiprakash B, Naira N (2011) Isolation & phytochemical investigation on leaves of Buchanania lanzan (Chironji). Ann Biol Res 2:469–473
Mehta SK, Mukherjee S, Jaiprakash B (2010) Preliminary phytochemical investigation on leaves of Buchanania lanzan (chironji). Int J Pharm Sci Rev Res 3(2):55–59
Mishra SN, Kumar D, Kumar B, Tiwari S (2021) Assessing impact of varying climatic conditions on distribution of Buchanania cochinchinensis in Jharkhand using species distribution modeling approach. Curr Res Environ Sustain 3:100025. https://doi.org/10.1016/j.crsust.2021.100025
Mohammad N, Dahayat A, Pardhi Y, Rajkumar M, Ansari SA, Shirin F (2022) Inferring genetic diversity and population structure of India’s National Teak (Tectona grandisL.f) Germplasm Bank. Genet Resour Crop Evol 69:1695–1705. https://doi.org/10.1007/s10722-021-01335-w
Mohammad N, Dahayat A, Yadav M, Shirin F, Ansari SA (2018) Genetic diversity and population structure of Litsea Glutinosa (Lour.) In Central India. Physiol Mol Biol Plants 24:655–663. https://doi.org/10.1007/s12298-018-0556-x
Mohammad N, Mahesh S, Jain YK, Ansari SA (2017) Effect of discrete (individual) and mixed (bulk) genomic DNA on genetic diversity estimates and population structure in Teak (Tectona grandisL.f). Indian J Exp Biol 55:44–48
Mohammad N, Mahesh S, Kumar P, Ansari SA (2012) Genotyping of Santalum album L. accessions through cross-species transferability of SSR markers of Santalum Austrocaledonicum and Santalum insulare. Sandalwood Res Newsl 1–4
Mondal M, Konar A, Halder S, Roy A, Dalal DD, Ghosh P (2024) An insight into the morphological, ethnomedicinal, phytochemical, and pharmaceutical properties of Buchanania Lanzan. J Med Plants Stud 12(1):11–17. https://doi.org/10.22271/plants.2024.v12.i1a.1620
Niratker C, Sailaja D (2018) Evaluation of genetic diversity of Chironji (Buchanania Lanzan) in Chhattisgarh. Int J Res Anal Rev 5:922–925
Patil NA, Udgire S, Shinde DR, Patil PD (2023) Green synthesis of gold nanoparticles using extract of Vitis vinifera, Buchanania Lanzan, Juglandaceae, phoenix dactylifera plants, and evaluation of antimicrobial activity. Chem Methodologies 7:15–27. https://doi.org/10.22034/chemm.2023.355289.1597
Peakall R, Smouse P (2012) GenAlEx 6.5: genetic analysis in Excel. Population genetic software for teaching and research– an update. Bioinformatics 28(19):2537–2539. https://doi.org/1093/bioinformatics/bts460
Pradhan D, Abdullah S, Pradhan RC (2020) Chironji (Buchanania Lanzan) fruit juice extraction using cellulase enzyme: modelling and optimization of process by artificial neural network and response surface methodology. J Food Sci Technol 58:1051–1060. https://doi.org/10.1007/s13197-020-04619-8
Prasad S (2020) Chironji (Buchanania Lanzan): a retreating valuable resource of central India. Int J Bioresource Sci 7:1–4. https://doi.org/10.30954/2347-9655.01.2020.1
Prevost A, Wilkinson MJ (1999) A new system of comparing PCR primers applied to ISSR ngerprinting of potato cultivars. Theor Appl Genet 98:107–112
Puri A, Sahai R, Singh KL, Saxena RP, Tandon JS, Saxena KC (2000) Immunostimulant activity of dry fruits and plant materials used in Indian traditional medical system for mothers after child birth and invalids. J Ethnopharmacol 71(1–2):89–92. https://doi.org/10.1016/S0378-8741(99)00181-6
Rai PK, Sharma DR, Sharma A (2015) Buchanania lanzan is a pharmacognostic miracle herb. Res J Pharmacognosy Phytochemistry 7(3):182–188. https://doi.org/10.5958/0975-4385.2015.00029.1
Santos VN, Gama RN, Santos CA (2021) Development and transferability of microsatellite loci for Spondias tuberosa (Anacardiaceae: Sapindales), a species endemic to the Brazilian semi-arid region. Genet Mol Res 20:1–8. https://doi.org/10.4238/gmr18778
Shepherd M, Cross M, Stoke LR, Scott LJ, Jones ME (2002) High-throughput DNA extraction from forest trees. Plant Mol Biol Rep 20:425–425. https://doi.org/10.1007/BF02772134
Shrivastava D, Saksena S (2024) Qualitative and quantitative determination of secondary metabolites and evaluation of Antiulcer Activity of Hydroalcoholic Extract of Buchanania Lanzan Leaf Extract. J Drug Deliv Ther 14:7. https://doi.org/10.22270/jddt.v14i7.6690
Siddiqui MZ, Chowdhury A, Prasad N, Thomas M (2014) Buchanania lazan: a species of enormous potentials. World J Pharm Sci 2(4):374–379
Soares TN, Santana LD, Oliveira LK, Telles MP, Collevatti RG (2013) Transferability and characterization of microsatellite loci in Anacardium Humile A. St. Hil. (Anacardiaceae). Genet Mol Res 12:3146–3149
Soldati MC, Inza MV, Fornes L, Zelener N (2014) Cross transferability of SSR markers to endangered Cedrela species that grow in Argentinean subtropical forests, as a valuable tool for population genetic studies. Biochem Syst Ecol 1:8–16. https://doi.org/10.1016/j.bse.2013.12.003
Srivastava A, Bishnoi SK, Sarkar PK (2017) Value addition in minor fruits of eastern India: an opportunity to generate rural employment. Fruits for Livelihood: Production Technology and Management Practices. Agrobios (India), Jodhpur, (India) pp. 395–417
Suresh D, Nethravathi PC, Kumar MP, Naika HR, Nagabhushana H, Sharma SC (2015) Chironji mediated facile green synthesis of ZnO nanoparticles and their photoluminescence, photodegradative, antimicrobial and antioxidant activities. Mater Sci Semiconduct Process 40:759–765. https://doi.org/10.1016/j.mssp.2015.06.088
Wang W, Li Z, Li Y (2014) Isolation and characterization of Microsatellite Markers for Cotinus coggygria Scop. (Anacardiaceae) by 454 pyrosequencing. Molecules 19:3813–3819. https://doi.org/10.3390/molecules19033813
Warokar AS, Ghante MH, Duragkar NJ, Bhusari KP (2010) Anti-inflammatory and antioxidant activities of methanolic extract of Buchanania Lanzan Kernel. Indian J Pharm Educ Res 44(4):363–368
WFO (2025) Buchanania cochinchinensis (Lour.) M.R.Almeida. Published on the Internet;http://www.worldfloraonline.org/taxon/wfo-0000573381. Accessed on: 03 Jan 2025
Yan Z, Wu F, Luo K, Zhao Y, Yan Q, Zhang Y, Wang Y, Zhang J (2017) Cross-species transferability of EST-SSR markers developed from the transcriptome of Melilotus and their application to population genetics research. Sci Rep 7(1):17959. https://doi.org/10.1038/s41598-017-18049-8
Author Information
Genetics and Tree Improvement Division, ICFRE-Tropical Forest Research Institute, Jabalpur, India