Examining the probiotic, antibacterial, cholesterol-lowering, β-galactosidase and cytotoxicity impacts of Lactococcus lactis isolated from cow milk
Research Articles | Published: 17 November, 2022
First Page: 1377
Last Page: 1383
Views: 4242
Keywords: Cow milk, Probiotic, n Lactoccocu lactisn , β-Galactosidase, Cholesterol assimilation, HT-29 cells
Abstract
Cow’s milk is an excellent source of calcium, iodine, vitamin B12, protein, and other minerals. Additionally, it includes whey and casein, which have been reported to contribute to blood pressure reduction, as well as magnesium, which is necessary for the formation of bones and muscles. In vitro testing was done on Lactococcus lactis strains that were obtained from cow milk. Various intestinal Gram positive and negative bacteria were effectively inhibited by this isolate, and it was able to survive 0.3% bile salt as well as gastric and intestinal conditions. With robust auto-aggregation and co-aggregation and increased hydrophobicity, destroy the pathogen and stop it from adhering to intestinal cells. Effective cholesterol assimilation by L. lactis raises the possibility that they can decrease cholesterol, and because they generate β-galactosidase, they can also be utilized to treat lactose intolerance. In vitro adhesion with HT-29 cells was tested for the presence of effective probiotic properties. The pathogen cannot adhere to intestinal cells because of these isolates, which adhere to HT-29 cells more successfully. Therefore, L. lactis can be used to create functional foods by exploiting its advantageous probiotic qualities.
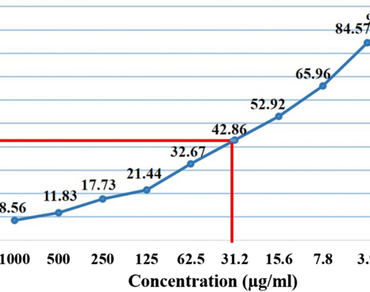
References
Adriana N, Zakłos-Szyda M, Rosicka-Kaczmarek J, Motyl I (2022) Anticancer potential of post-fermentation media and cell extracts of probiotic strains: an in vitro study. Cancers (basel) 14(7):1853
Asadi A, Razavi S, Talebi M, Gholami M (2019) A review on anti-adhesion therapies of bacterial diseases. Infection 47:13–23. https://doi.org/10.1007/s15010-018-1222-5
Dashkevicz MP, Feighner SD (1989) Development of a differential medium for bile salt hydrolase-active Lactobacillus spp. Appl Environ Microbiol 55:11–16
De Valdez GF, De Taranto MP (2001) Probiotic properties of Lactobacilli: cholesterol reduction and bile salt hydrolase activity. In: Spencer JFT, de Spencer ALR (eds) Food microbiology protocols. Humana Press, New Jersey, pp 173–182
Fesseha H, Demlie T, Mathewos M, Eshetu E (2021) Effect of Lactobacillus species probiotics on growth performance of dual-purpose chicken. Vet Med Res Rep 12:75–83
Gao Y, Li D, Liu S, Liu Y (2012) Probiotic potential of L. sake C2 isolated from traditional Chinese fermented cabbage. Eur Food Res Technol 234:45–51
Kouhi F, Mirzaei H, Nami Y, Khandaghi J, Javadi A (2022) Potential probiotic and safety characterisation of enterococcus bacteria isolated from indigenous fermented motal cheese. Int Dairy J 126:105247. https://doi.org/10.1016/j.idairyj.2021.105247
Kumar M, Nagpal R, Kumar R, Hemalatha R, Verma V, Kumar A, Chakraborty C, Singh B, Marotta F, Jain S, Yadav H (2012) Cholesterol-lowering probiotics as potential biotherapeutics for metabolic diseases. Exp Diabetes Res. https://doi.org/10.1155/2012/902917
Mahsa S, Bahman P, Atefeh M, Mohammad AH, Daniel Elieh AK, Yousef N (2022) Screening of potential probiotic lactic acid bacteria with antimicrobial properties and selection of superior bacteria for application as biocontrol using machine learning models. LWT Food Sci Technol 162:113471. https://doi.org/10.1016/j.lwt.2022.113471
Mazlumi A, Panahi B, Amin Hejazi M, Nami Y (2022) Probiotic potential characterization and clustering using unsupervised algorithms of lactic acid bacteria from saltwater fish samples. Sci Rep 12:11952. https://doi.org/10.1038/s41598-022-16322-z
Millette M, Luquet FM, Ruiz MT, Lacroix M (2008) Characterization of probiotic properties of Lacto bacillus strains. Dairy Sci Technol 88(6):695–705. https://doi.org/10.1051/dst:2008018
Mokoena MP (2017) Lactic acid bacteria and their bacteriocins: classification, biosynthesis and applications against uropathogens: a mini-review. Molecules 22:1255. https://doi.org/10.3390/molecules22081255
Nami Y, Bakhshayesh RV, Manafi M, Hejazi MA (2019) Hypocholesterolaemic activity of a novel autochthonous potential probiotic Lactobacillus plantarum YS5 isolated from yogurt. LWT Food Sci Technol. https://doi.org/10.1016/j.lwt.2019.05.057
Nami Y, Panahi B, Jalaly HM, Bakhshayesha RV, Amin Hejazi M (2020) Application of unsupervised clustering algorithm and heat-map analysis for selection of lactic acid bacteria isolated from dairy samples based on desired probiotic properties. LWT Food Sci Technol 118:108839. https://doi.org/10.1016/j.lwt.2019.108839
Nivetha A, Mohana Srinivasan V (2017) Mini review on role of β-galactosidase in lactose intolerance. Mater Sci Eng 263:022046. https://doi.org/10.1088/1757-899X/263/2/022046
Nur SA, Noor HMdZ, Tengku Haziyamin TAH (2017) Lactococcus Lactis Strain A5 producing nisin-like bacteriocin active against gram positive and negative bacteria. Trop Life Sci Res 28(2):107–118. https://doi.org/10.21315/tlsr2017.28.2.8
Osmanagaoglu O, Kiran F, Ataoglu H (2010) Evaluation of in vitro probiotic potential of Pediococcus pentosaceus OZF isolated from human breast milk. Probiotics Antimicrobial Proteins 2:162–174
Philip AV, Umar TM, Bulus T (2017) Isolation and identification of lactic acid bacteria with probiotic potential from fermented cow milk (Nono) in Unguwar Rimi Kaduna State Nigeria. Am J Mol Biol 7:99–106
Ramirez J, Guarner F, Fernandez LB, Maruy A, Sdepanian VL, Cohen H (2020) Antibiotics as major disruptors of Gut microbiota. Front Cell Infect Microbiol 10:572912. https://doi.org/10.3389/fcimb.2020.572912
Ranita R, Monalisa T, Gianfranco D, Vishvanath T (2017) Strategies for combating bacterial biofilms: a focus on anti-biofilm agents and their mechanisms of action. Virulence. https://doi.org/10.1080/21505594.2017.1313372
Rodriguez E, Arques JL, Rodriguez R, Peiroten A, Landete JM, Medina M (2012) Antimicrobial properties of probiotic strains isolated from breast-fed infants. J Function Foods 4:542–551
Singhal N, Maurya AK, Mohanty S, Kumar M, Singh Virdi J (2019) Evaluation of bile salt hydrolases, cholesterol-lowering capabilities, and probiotic potential of Enterococcus faecium isolated from rhizosphere. Front Microbiol 10:1567. https://doi.org/10.3389/fmicb.2019.01567
Suba S, Vijayakumar S, Nilavukkarasi M, Vidhya E, Punitha VN (2022) Eco synthesized silver nanoparticles as a next generation of nanoproduct in multidisciplinary applications. Environ Chem Ecotoxicol 4:13–19
Suzuki A, Miwa S (2021) Antimicrobial Activity of Lactococcus lactis subsp. lactis Isolated from a Stranded Cuvier’s Beaked Whale (Ziphius cavirostris) against Gram-Positive and -Negative Bacteria. Microorganisms 9:243. https://doi.org/10.3390/microorganisms9020243
Terpou A, Papadaki A, Lappa IK, Kachrimanidou V, Bosnea LA, Kopsahelis N (2019) Probiotics in food systems: significance and emerging strategies towards improved viability and delivery of enhanced beneficial value. Nutrients 11:1591. https://doi.org/10.3390/nu11071591
Umran A, Martin L, Arno C, Olde Damink SWM, Carsten B, Schaap FG (2022) New kids on the block: bile salt conjugates of microbial origin. Metabolites 12:176. https://doi.org/10.3390/metabo12020176
Vidhyasagar V, Jeevaratnam K (2013) Evaluation of Pediococcus pentosaceus strains isolated from idly batter for probiotic properties in vitro. Journal of Functional Foods 5:235–243
Vinderola CG, Reinheimer JA (2003) Lactic acid starter and probiotic bacteria: a comparative ‘“in vitro”’ study of probiotic characteristics and biological barrier resistance. Food Res Int 36:895–904
Wan Norlina WA, Julia O, Tan Say K, Salwani T, Ismail T (2019) Hemolyzed specimens: major challenge for identifying and rejecting specimens in clinical laboratories. Oman Med J 34(2):94–98
Yeshambel T, Tadesse D, Haben F, Mesfin M (2021) Isolation and identification of lactic acid bacteria from cow milk and milk products. Sci World J. https://doi.org/10.1155/2021/4697445
Yijing Y, Fitore R, Khosrow A (2019) The role of the gut microbiota in lipid and lipoprotein metabolism. J Clin Med 8:2227. https://doi.org/10.3390/jcm8122227
Monteagudo-Mera A, Rodríguez-Aparicio L, Rúa J, Martínez-Blanco H, Navasa N, García-Armesto MR, Ferrero MÁ (2012) In vitro evaluation of physiological probiotic properties of different lactic acid bacteria strains of dairy and human origin. J Funct Foods 4(2):531–541. https://doi.org/10.1016/j.jff.2012.02.014
CLSI (2011) Performance standards for antimicrobial susceptibility testing, 21st informational supplement. CLSI document M100-S21. Clinical and Laboratory Standards Institute, Wayne
Author Information
Computational Phytochemistry Laboratory, PG and Research Department of Botany and Microbiology, A.V.V.M. Sri Pushpam College (Autonomous) (Affiliated to Bharathidasan University), Poondi, India