Extraction, Purification and Characterization of Peroxidase form Bacopa monnieri from Ranchi
Research Article | Published: 11 January, 2019
First Page: 123
Last Page: 125
Views: 3890
Keywords: Bacopa monnieri, oxidoreductase, peroxidase, immobilization, wound healing
Abstract
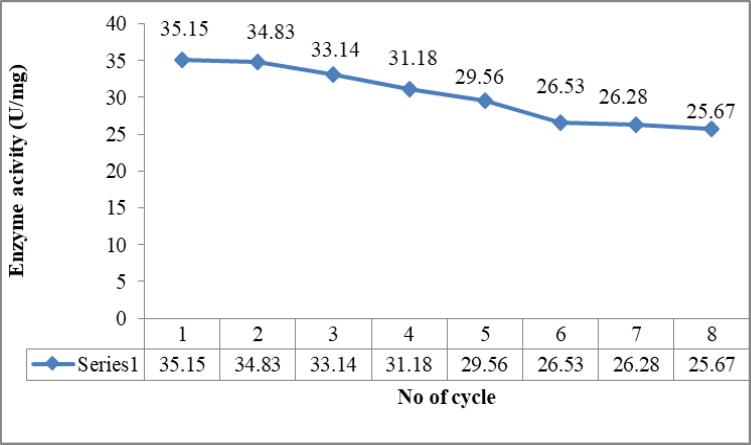
Peroxidase is an oxidoreductase enzyme catalysing many oxidation reduction reactions in presence of iron as cofactor. Main function of peroxidase is the scavenging of reactive oxygen species to reduce oxidative stress and damage. It is universally present in living organisms such as bacteria, fungi, plants and animals. Peroxidases also contribute in lignin biosynthesis, hormone generation, and detoxification by removal of hydrogen peroxide from cell organelles and oxidation of toxins. Industrially, peroxidases are also important for the removal of phenols from industrial wastes, manufacturing of adhesives, and computer chips. On the other hand Bacopa monnieri is one of the well known plants with immense importance since ages. The present work included both the enzyme and plant resource as peroxidase enzyme was extract from Bacopa monnieri. After screening different parts of Bacopa monnieri fresh leaves were used for partial purification peroxidases enzyme. Crude enzyme extracted from sample was partially purified by using ammonium sulfate precipitation which was further used for immobilization. As result of purification peroxidase activity was enhanced from 63.51 U/mg of protein to 123.87U/mg. Optimization of purification process exhibited increase in enzyme recovery. Moreover immobilized enzyme also showed remarkable stability and reusability with after immobilization which suggested the suture applicability.
References
- Colonna S, Gaggero N, Richelmi C and Pasta P (1999) Recent biotechnological developments in the use of peroxidases. Trends Biotech 17(4): 163-168.
- Hamid M (2009) Potential applications of peroxidases. Food Chem 115(4): 1177-1186.
- Asada K (1992) Ascorbate peroxidase–a hydrogen peroxide‐scavenging enzyme in plants. Physiologia Plant 85(2): 235-241.
- Hiraga S, Sasaki K, Ito H, Ohashi Y and Matsui H (2001) A Large Family of Class III Plant Peroxidases, Plant and Cell Physiol 42(5): 462–468.
- Bacopa Monnieri (2008) Germplasm Resources Information Network (GRIN). Agricultural Research Service (ARS), United States Department of Agriculture (USDA). Retrieved 2008-03-13.
- Schuller DJ, Ban N, Huystee RB, McPherson and Poulos TL (1996) The crystal structure of peanut peroxidase. Structure 4(3):311-321.
- Chance B and Maehly AC (1995) Assay of catalases and peroxidases. Methods in Enzymology (Elsevier), 2:764-775.
- Lowry OH, NJ Rosbrough AL Farr and RJ Randall J. Biol. Chem. 193: 265. 1951.
- Nadaroglu H, Celebi N, Demir N and Demir Y (2013) Purification and characterisation of a plant peroxidase from rocket (Eruca vesicaria sbsp. Sativa) (Mill.) (syn. E. sativa) and effects of some chemicals on peroxidase activity in vitro, African J Agric Res 8 (21): 2520-2528.
- Pinto MST, Ribeiro JM, Araujo FP, Melo NF and Fernandes KVS (2015) Purification and characterization of a peroxidase present in xilopodium exsudates of umbu plants (Spondias tuberosa A.), African J Agric Res 14 (21): 1838-1845.
- Galende PP, De María CG, Arellano JB, Roig MG and Shnyrov VL (2016) Study on Extraction, Purification and Characterization of a Novel Peroxidase from White Spanish Broom (Cytisus multiflorus). Int J Plant Biol Res 4(1): 1052.
- Kishore L, Kaur N and Singh R (2016) Renoprotective effect of Bacopa monnieri via inhibition of advanced glycation end products and oxidative stress in STZ-nicotinamide-induced diabetic nephropathy. Renal failure 38(9): 1528-1544.
Author Information
Laboratory of Plant Physiology and Biotechnology, University Department of Botany, Ranchi University, Ranchi- 834008, Jharkhand, India