Green biosynthesis of silver nanoparticles and impact on growth, chlorophyll, yield and phytotoxicity of Phaseolus vulgaris L
Research Articles | Published: 03 August, 2020
First Page: 648
Last Page: 657
Views: 4020
Keywords: Chlorophyll, Silver nanoparticles, Phaseolus vulgaris , Phytotoxicity
Abstract
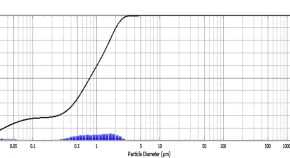
Silver nanoparticles (Ag NPs) are used worldwide for research experiments in various fields. The green biosynthesis method used for the synthesis of Ag NPs and its characterization was completed by PSA (particle size analyzer), SEM (scanning electron microscopy), XRD (X-ray diffraction) and TEM (transmission electron microscopy). The objective of this research study was to find out the effect of biosynthesis Ag NPs on seed germination, root elongation, shoot growth, photosynthetic pigments (chlorophyll a, chlorophyll b and carotenoids), plant improvement and yield production, etc. of Phaseolus vulgaris L. Six different concentrations such as 0, 15, 30, 60, 120, 240 and 480 mg L−1 were applied after seed sterilization by seed treatment method. Morphological parameters and phytochemicals of plants treated at different concentrations were compared with control plants. Chlorophyll increase in phytochemical activity was observed in seeds treated with low concentrations of Ag nanoparticles. While higher concentrations showed greater toxicity, but root elongation was observed in seeds treated with 120 mg L−1 concentration.
References
- Al-Huqail AA, Hatata MM, Al-Huqail AA, Ibrahim MM (2018) Preparation, characterization of silver phyto nanoparticles and their impact on growth potential of Lupinus termis L. seedlings. Saudi J Biol Sci 25(2):313–319
- Castiglione MR, Giorgetti L, Geri C, Cremonini R (2011) The effects of nano-TiO2 on seed germination, development and mitosis of root tip cells of Vicia narbonensis L. and Zea mays L. J Nanopart Res 13(6):2443–2449
- Castro-González CG, Sánchez-Segura L, Gómez-Merino FC, Bello-Bello JJ (2019) Exposure of Stevia (Stevia rebaudiana B.) to Silver nanoparticles in vitro: transport and accumulation. Sci Rep 9(1):1–10
- Chaurasiya U, Patel S, Agnihotri RK (2018) Iron induce different changes at different stages of the development of Brassica nigra. Discov Nat 12:77–81
- Das A, Kamle M, Bharti A, Kumar P (2019) Nanotechnology and it’s applications in environmental remediation: an overview. Vegetos 32(3):227–237
- El-Batal AI, Gharib F, Ghazi SM, Hegazi AZ, Hafz A (2016) Physiological responses of two varieties of common bean (Phaseolus vulgaris L.) to foliar application of silver nanoparticles. Nanomater Nanotechnol 6:13
- Farhadi K, Forough M, Molaei R, Hajizadeh S, Rafipour A (2012) Highly selective Hg2+ colorimetric sensor using green synthesized and unmodified silver nanoparticles. Sens Actuators B Chem 161(1):880–885
- Gardea-Torresdey JL, Gomez E, Peralta-Videa JR, Parsons JG, Troiani H, Jose-Yacaman M (2003) Alfalfa sprouts: a natural source for the synthesis of silver nanoparticles. Langmuir 19(4):1357–1361
- Gautam S, Misra P, Shukla PK, Ramteke PW (2016) Effect of copper oxide nanoparticle on the germination, growth and chlorophyll in Soybean (Glycine max (L.). Vegetos 29:157–160
- Geisler-Lee J, Brooks M, Gerfen JR, Wang Q, Fotis C, Sparer A, Geisler M (2014) Reproductive toxicity and life history study of silver nanoparticle effect, uptake and transport in Arabidopsis thaliana. Nanomaterials 4(2):301–318
- Gupta SD, Agarwal A, Pradhan S (2018) Phytostimulatory effect of silver nanoparticles (AgNPs) on rice seedling growth: an insight from antioxidative enzyme activities and gene expression patterns. Ecotoxicol Environ Saf 161:624–633
- Hong F, Zhou J, Liu C, Yang F, Wu C, Zheng L, Yang P (2005) Effect of nano-TiO2 on photochemical reaction of chloroplasts of spinach. Biol Trace Elem Res 105(1–3):269–279
- Jośko I, Oleszczuk P (2013) Influence of Soil type and Environmental conditions on ZnO, TiO2 and Ni nanoparticles phytotoxicity. Chemosphere 92(1):91–99
- Kaegi R, Voegelin A, Sinnet B, Zuleeg S, Hagendorfer H, Burkhardt M, Siegrist H (2011) Behavior of metallic silver nanoparticles in a pilot wastewater treatment plant. Environ Sci Technol 45(9):3902–3908
- Karimi N, Minaei S, Almassi M, Shahverdi AR (2012) Application of silver nano-particles for protection of seeds in different Soils. Afr J Agric Res 7(12):1863–1869
- Khiew P, Chiu W, Tan T, Radiman S, Abd-Shukor R, Chia CH (2011) Capping effect of palm-oil based organometallic ligand towards the production of highly monodispersed nanostructured material. In: Palm oil: nutrition, uses and impacts, pp 189–219
- Kovačević DB, Maras M, Barba FJ, Granato D, Roohinejad S, Mallikarjunan K, Putnik P (2018) Innovative technologies for the recovery of phytochemicals from Stevia rebaudiana Bertoni leaves: a review. Food Chem 268:513–521
- Kushwah KS, Patel S (2019) Effect of titanium dioxide nanoparticles (TiO2 NPs) on Faba bean (Vicia faba L.) and induced asynaptic mutation: a meiotic study. J Plant Growth Regul 1–12
- Kushwah KS, Verma RC, Patel S, Jain NK (2018) Colchicine induced polyploidy in Chrysanthemum carinatum L. J Phylogenet Evol Biol 6(193):2
- Kutoš T, Golob T, Kač M, Plestenjak A (2003) Dietary fibre content of dry and processed beans. Food Chem 80(2):231–235
- Lalitha A, Subbaiya R, Ponmurugan P (2013) Green synthesis of Silver nanoparticles from leaf extract Azhadirachta indica and to study its anti-bacterial and antioxidant property. Int J Curr Microbiol Appl Sci 2(6):228–235
- Mahakham W, Sarmah AK, Maensiri S, Theerakulpisut P (2017) Nanopriming technology for enhancing germination and starch metabolism of aged rice seeds using phytosynthesized Silver nanoparticles. Sci Rep 7(1):1–21
- Maiti S, El-Fahime E, Benaissa M, Kaur Brar S (2015) Nano-ecotoxicology of natural and engineered nanoparticles for plants. Nanomater Environ 469–485
- Mehta CM, Srivastava R, Arora S, Sharma AK (2016) Impact assessment of silver nanoparticles on plant growth and soil bacterial diversity. 3 Biotech 6(2):254
- Miao AJ, Schwehr KA, Xu C, Zhang SJ, Luo Z, Quigg A, Santschi PH (2009) The algal toxicity of silver engineered nanoparticles and detoxification by exopolymeric substances. Environ Pollut 157(11):3034–3041
- Musante C, White JC (2012) Toxicity of silver and copper to Cucurbita pepo: differential effects of nano and bulk-size particles. Environ Toxicol 27(9):510–517
- Palanisamy NK, Ferina N, Amirulhusni AN, Mohd-Zain Z, Hussaini J, Ping LJ, Durairaj R (2014) Antibiofilm properties of chemically synthesized silver nanoparticles found against Pseudomonas aeruginosa. J Nanobiotechnol 12(1):2
- Pradas del Real AE, Vidal V, Carrière M, Castillo-Michel H, Levard C, Chaurand P, Sarret G (2017) Silver nanoparticles and wheat roots: a complex interplay. Environ Sci Technol 51(10):5774–5782
- Prasad TN, Adam S, Rao PV, Reddy BR, Krishna TG (2016) Size dependent effects of antifungal phytogenic silver nanoparticles on germination, growth and biochemical parameters of rice (Oryza sativa L.), maize (Zea mays L.) and peanut (Arachis hypogaea L.). IET Nanobiotechnol 11(3):277–285
- Puchana-Rosero MJ, Adebayo MA, Lima EC, Machado FM, Thue PS, Vaghetti JC, Gutterres M (2016) Microwave-assisted activated carbon obtained from the sludge of tannery-treatment effluent plant for removal of leather dyes. Colloids Surf A 504:105–115
- Racuciu M, Creanga DE (2007) TMA-OH coated magnetic nanoparticles internalized in vegetal tissue. Rom J Phys 52(3/4):395
- Razzaq A, Ammara R, Jhanzab HM, Mahmood T, Hafeez A, Hussain S (2016) A novel nanomaterial to enhance growth and yield of wheat. J Nanosci Technol 2(1):55–58
- Rezvani N, Sorooshzadeh A, Farhadi N (2012) Effect of nano-silver on growth of saffron in flooding stress. World Acad Sci Eng Technol 6(1):517–522
- Saeideh N, Rashid J (2014) Effect of Silver nanoparticles and Pb (NO3)2 on the yield and chemical composition of mung bean (Vigna radiata). J Stress Physiol Biochem 10(1)
- Salama HM (2012) Effects of Silver nanoparticles in some crop plants, common bean (Phaseolus vulgaris L.) and corn (Zea mays L.). Int Res J Biotechnol 3(10):190–197
- Sharma P, Bhatt D, Zaidi MGH, Saradhi PP, Khanna PK, Arora S (2012) Silver nanoparticle-mediated enhancement in growth and antioxidant status of Brassica juncea. Appl Biochem Biotechnol 167(8):2225–2233
- Singh M, Singh S, Prasad S, Gambhir IS (2008) Nanotechnology in medicine and antibacterial effect of silver nanoparticles. Digest J Nanomater Biostruct 3(3):115–122
- Sosan A, Svistunenko D, Straltsova D, Tsiurkina K, Smolich I, Lawson T, Colbeck I (2016) Engineered silver nanoparticles are sensed at the plasma membrane and dramatically modify the physiology of Arabidopsis thaliana plants. Plant J 85(2):245–257
- Sumanta N, Haque CI, Nishika J, Suprakash R (2014) Spectrophotometric analysis of chlorophylls and carotenoids from commonly grown fern species by using various extracting solvents. Res J Chem Sci 4(9):63–69
- Syu YY, Hung JH, Chen JC, Chuang HW (2014) Impacts of size and shape of silver nanoparticles on Arabidopsis plant growth and gene expression. Plant Physiol Biochem 83:57–64
- Thuesombat P, Hannongbua S, Akasit S, Chadchawan S (2014) Effect of silver nanoparticles on rice (Oryza sativa L. cv. KDML 105) seed germination and seedling growth. Ecotoxicol Environ Saf 104:302–309
- Urwat U, Zargar SM, Manzoor M, Ahmad SM, Ganai NA, Murtaza I, Khan I, Nehvi FA (2019) Morphological and biochemical responses of Phaseolus vulgaris L. to mineral stress under in vitro conditions. Vegetos 32(3):431–438
- Verma DK, Patel S, Kushwah KS (2020) Synthesis of Titanium dioxide (TiO2) nanoparticles and impact on morphological changes, seeds yield and phytotoxicity of Phaseolus vulgaris L. Trop Plant Res 7(1):158–170
- Vivek R, Thangam R, Muthuchelian K, Gunasekaran P, Kaveri K, Kannan S (2012) Green biosynthesis of silver nanoparticles from Annona squamosa leaf extract and its in vitro cytotoxic effect on MCF-7 cells. Process Biochem 47(12):2405–2410
- Wesołowska A, Jadczak P, Kulpa D, Przewodowski W (2019) Gas chromatography-mass spectrometry (GC-MS) analysis of essential oils from AgNPs and AuNPs elicited Lavandula angustifolia in vitro cultures. Molecules 24(3):606
- Yang L, Watts DJ (2005) Particle surface characteristics may play an important role in phytotoxicity of alumina nanoparticles. Toxicol Lett 158(2):122–132
Author Information
School of Studies in Botany, Jiwaji University, Gwalior, India