Improvements in the efficiency of Agrobacterium rhizogenes-mediated transformation of Oroxylum indicum for induction of hairy roots
Research Articles | Published: 24 September, 2022
First Page: 929
Last Page: 938
Views: 16412
Keywords: Oroxylum indicum , Agrobacterium rhizogenes ATCC 15834, Hairy root, Anti-browning agents, Flavonoids
Abstract
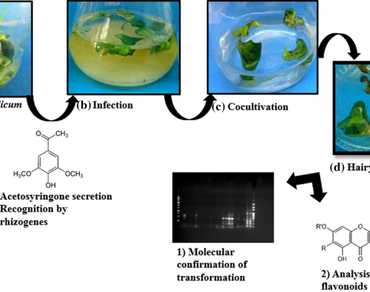
Oroxylum indicum (Shyonak) is used as a medicinal herb in the ethnomedicinal system of Asia for prevention and treatment of diseases like respiratory disorder, gastric ulcers, diabetes, cancer, rheumatoid problems, etc. Prior studies have linked the pharmacological properties to flavonoids present in seeds, stems, and root bark. The current research aims to develop a long-term stable system of transgenic hairy roots of O. indicum using Agrobacterium rhizogenes strain ATCC 15834. The best medium, explant type, infection time, co-cultivation period, and anti-browning chemicals were optimized during the hairy root induction. The maximum transformation frequency (49.16 ± 2.44%) was documented for leaf explant after 30 days of inoculation on a semi-solid B5 medium with cefotaxime (200 mg/l), at 48-h co-cultivation period followed by a 2-h infection phase. Explant browning also hindered biomass growth. Hence, different anti-oxidants were used to control cell biomass browning. A transformation frequency up to 65.8% was obtained in B5 media with cysteine (3.71 mM). Successful transformation of the hairy root lines was confirmed using PCR amplification using A. rhizogenes virD, aux1, rolA, rolB, and rolC genes specific primers. Subsequently, in vitro production of four flavonoids viz., baicalein, hispidulin, scutellarin, and biochanin-A, were quantified in hairy root culture of O. indicum and compared with roots of two months old greenhouse cultivated plantlets and roots of in vitro regenerated plants. Higher quantities of baicalein, hispidulin, and scutellarin (viz., 11.47 ± 1.5 μg/g DW, 10.45 ± 0.27 μg/g DW, and 24.75 ± 0.85 μg/g DW, respectively) were quantified in the hairy root cultures of O. indicum.
Graphical abstract

References
Amani S, Mohebodini M, Khademvatan S, Jafari M, Kumar V (2021) Piriformospora indica based elicitation for overproduction of phenolic compounds by hairy root cultures of Ficus carica. J Biotechnol 327:43–53. https://doi.org/10.1016/j.jbiotec.2020.12.015
Baek S, Han J-E, Ho T-T, Park S-Y (2022) Development of hairy root cultures for biomass and triterpenoid production in Centella asiatica. Plants 11(2):148. https://doi.org/10.3390/PLANTS11020148
Balasubramanian M, Anbumegala M, Surendran R, Arun M, Shanmugam G (2018) Elite hairy roots of Raphanus sativus (L.) as a source of antioxidants and flavonoids. 3 Biotech 8(2):128. https://doi.org/10.1007/s13205-018-1153-y
Begum M, Islam A, Begum R, Uddin MS, Rahman MS, Alam S, Akter W, Das M, Rahman MS, Imon AHMR (2019) Ethnopharmacological inspections of organic extract of oroxylum indicum in rat models: a promising natural gift. Evid Based Complement Altern Med. https://doi.org/10.1155/2019/1562038
Bhandari S, Sinha S, Nailwal TK, Thangadurai D (2022) Nanotechnology: an approach for enhancement of plant system in terms of tissue culture. Biogen Nanomater. https://doi.org/10.1201/9781003277149-9
Brijwal L, Tamta S (2015) Agrobacterium rhizogenes mediated hairy root induction in endangered Berberis aristata DC. Springerplus 4(1):443. https://doi.org/10.1186/s40064-015-1222-1
Bruni R, Sacchetti G (2009) Factors affecting polyphenol biosynthesis in wild and field grown St. John’s Wort (Hypericum perforatum L. Hypericaceae/Guttiferae). Molecules 14(2):682–725. https://doi.org/10.3390/molecules14020682
Bulgakov VP, Khodakovskaya MV, Labetskaya NV, Chernoded GK, Zhuravlev YN (1998) The impact of plant rolC oncogene on ginsenoside production by ginseng hairy root cultures. Phytochemistry 49(7):1929–1934. https://doi.org/10.1016/S0031-9422(98)00351-3
Bulgakov VP, Tchernoded GK, Mischenko NP, Khodakovskaya MV, Glazunov VP, Radchenko SV, Zvereva EV, Fedoreyev SA, Zhuravlev YN (2002) Effect of salicylic acid, methyl jasmonate, ethephon and cantharidin on anthraquinone production by Rubia cordifolia callus cultures transformed with the rolB and rolC genes. J Biotechnol 97(3):213–221. https://doi.org/10.1016/S0168-1656(02)00067-6
Chayjarung P, Poonsap W, Pankaew C, Inmano O, Kongbangkerd A, Limmongkon A (2021) Using a combination of chitosan, methyl jasmonate, and cyclodextrin as an effective elicitation strategy for prenylated stilbene compound production in Arachis hypogaea L hairy root culture and their impact on genomic DNA. Plant Cell, Tissue and Organ Culture (PCTOC) https://doi.org/10.1007/s11240-021-02112-4
Chilton MD, Tepfer DA, Petit A, David C, Casse Delbart F, Tempé J (1982) Agrobacterium rhizogenes inserts T-DNA into the genomes of the host plant root cells. Nature 295(5848):432–434. https://doi.org/10.1038/295432a0
Condori J, Sivakumar G, Hubstenberger J, Dolan MC, Sobolev VS, Medina-Bolivar F (2010) Induced biosynthesis of resveratrol and the prenylated stilbenoids arachidin-1 and arachidin-3 in hairy root cultures of peanut: Effects of culture medium and growth stage. Plant Physiol Biochem PPB 48(5):310–318. https://doi.org/10.1016/j.plaphy.2010.01.008
Das BB, Sen N, Roy A, Dasgupta SB, Ganguly A, Mohanta BC, Dinda B, Majumder HK (2006) Differential induction of Leishmania donovani bi-subunit topoisomerase I-DNA cleavage complex by selected flavones and camptothecin: activity of flavones against camptothecin-resistant topoisomerase I. Nucleic Acids Res 34(4):1121–1132. https://doi.org/10.1093/nar/gkj502
Dinda B, SilSarma I, Dinda M, Rudrapaul P (2015) Oroxylum indicum (L.) Kurz, an important Asian traditional medicine: From traditional uses to scientific data for its commercial exploitation. J Ethnopharmacol 161:255–278. https://doi.org/10.1016/j.jep.2014.12.027
Fang L, Yang T, Medina-Bolivar F (2020) Production of prenylated stilbenoids in hairy root cultures of peanut (Arachis hypogaea) and its wild relatives A. ipaensis and A. duranensis via an optimized elicitation procedure. Molecules. https://doi.org/10.3390/MOLECULES25030509
Fattahi F, Shojaeiyan A, Palazon J, Moyano E, Torras-Claveria L (2021) Methyl-β-cyclodextrin and coronatine as new elicitors of tropane alkaloid biosynthesis in Atropa acuminata and Atropa belladonna hairy root cultures. Physiol Plant 172(4):2098–2111. https://doi.org/10.1111/PPL.13444
Gharari Z, Bagheri K, Danafar H, Sharafi A (2020) Enhanced flavonoid production in hairy root cultures of Scutellaria bornmuelleri by elicitor induced over-expression of MYB7 and FNSП2 genes. Plant Physiol Biochem 148:35–44. https://doi.org/10.1016/j.plaphy.2020.01.002
Giri A, Giri CC, Dhingra V, Narasu ML (2001) Enhanced podophyllotoxin production from agrobacterium rhizogenes transformed cultures of podophyllum hexandrum. Nat Prod Lett 15(4):229–235. https://doi.org/10.1080/10575630108041286
Gutierrez-Valdes N, Häkkinen ST, Lemasson C, Guillet M, Oksman-Caldentey KM, Ritala A, Cardon F (2020) Hairy root cultures—a versatile tool with multiple applications. Front Plant Sci. https://doi.org/10.3389/fpls.2020.00033
Hidalgo D, Georgiev M, Marchev A, Bru-Martínez R, Cusido RM, Corchete P, Palazon J (2017) Tailoring tobacco hairy root metabolism for the production of stilbenes. Sci Rep 7(1):17976. https://doi.org/10.1038/s41598-017-18330-w
Huang SH, Vishwakarma RK, Lee TT, Chan HS, Tsay HS (2014) Establishment of hairy root lines and analysis of iridoids and secoiridoids in the medicinal plant Gentiana scabra. Bot Stud 55(1):1–8. https://doi.org/10.1186/1999-3110-55-17
Jia H, Chen J, Zhang L, Zhang L (2022) The first report on transgenic hairy root induction from the stem of tung tree ( Vernicia fordii). Plants (Basel, Switzerland) 11(10):1315. https://doi.org/10.3390/PLANTS11101315
Jones AMP, Saxena PK (2013) Inhibition of phenylpropanoid biosynthesis in artemisia annua L.: A Novel approach to reduce oxidative browning in plant tissue culture. PLoS One 8(10):76802. https://doi.org/10.1371/JOURNAL.PONE.0076802
Joshi N (2016) Micro/macro propagation of Oroxylum indicum (L.) vent and its antioxidant activity analysis [Kumaun University, Nainital]. http://hdl.handle.net/10603/213498
Li S, Cong Y, Liu Y, Wang T, Shuai Q, Chen N, Gai J, Li Y (2017) Optimization of agrobacterium-mediated transformation in soybean. Front Plant Sci 8:246. https://doi.org/10.3389/FPLS.2017.00246/BIBTEX
Matveeva TV, Sokornova SV (2016) Agrobacterium rhizogenes-mediated transformation of plants for improvement of yields of secondary metabolites. Bioproc Plant Vitro Syst. https://doi.org/10.1007/978-3-319-32004-5_18-1
Mehrotra S, Kukreja AK, Khanuja SPS, Mishra BN (2008) Genetic transformation studies and scale up of hairy root culture of Glycyrrhiza glabra in bioreactor. Electron J Biotechnol. https://doi.org/10.2225/vol11-issue2-fulltext-6
Mehrotra S, Srivastava V, Ur Rahman L, Kukreja AK (2015) Hairy root biotechnology–indicative timeline to understand missing links and future outlook. Protoplasma 252(5):1189–1201. https://doi.org/10.1007/s00709-015-0761-1
Meng D, Yang Q, Dong B, Song Z, Niu L, Wang L, Cao H, Li H, Fu Y (2019) Development of an efficient root transgenic system for pigeon pea and its application to other important economically plants. Plant Biotechnol J 17(9):1804–1813. https://doi.org/10.1111/PBI.13101
Palazón J, Cusidó RM, Roig C, Piñol MT (1998) Expression of the rolC gene and nicotine production in transgenic roots and their regenerated plants. Plant Cell Rep 17(5):384–390. https://doi.org/10.1007/S002990050411
Patel R, Mahakur B, Mitra D, Barik DP (2021) Preliminary hairy root induction in aerial plant parts of Paederia foetida using Agrobacterium rhizogenes. Int J Bot Stud 6(3):135–138. http://www.botanyjournals.com/archives/2021/vol6/issue3/6-2-56
Qin S, Liu Y, Yan J, Lin S, Zhang W, Wang B (2022) An optimized tobacco hairy root induction system for functional analysis of nicotine biosynthesis-related genes. Agronomy 12(2):348. https://doi.org/10.3390/AGRONOMY12020348
Rahamooz-Haghighi S, Bagheri K, Sharafi A, Danafar H (2020) Establishment and elicitation of transgenic root culture of Plantago lanceolata and evaluation of its anti-bacterial and cytotoxicity activity. Prepar Biochem Biotechnol 51(3):207–224. https://doi.org/10.1080/10826068.2020.1805757
Rekha K, Thiruvengadam M (2017) Secondary metabolite production in transgenic hairy root cultures of cucurbits. In Reference Series in Phytochemistry (pp. 267–293). Springer Science and Business Media B.V. https://doi.org/10.1007/978-3-319-28669-3_6
Ron M, Kajala K, Pauluzzi G, Wang D, Reynoso MA, Zumstein K, Garcha J, Winte S, Masson H, Inagaki S, Federici F, Sinh N, Deal RB, Bailey-Serres J, Brady SM (2014) Hairy root transformation using Agrobacterium rhizogenes as a tool for exploring cell type-specific gene expression and function using tomato as a model. Plant Physiol 166(2):455–469. https://doi.org/10.1104/pp.114.239392
Samadi A, Carapetian J, Heidari R, Jafari M, Gorttapeh AH (2012) Hairy root induction in Linum mucronatum ssp mucronatum, an anti-tumor lignans producing plant. Notulae Botanicae Horti Agrobotanici Cluj-Napoca, 40(1): 125–131 https://doi.org/10.15835/nbha4017312
Sharma S, Chhimwal N, Bhatt KK, Sharma AK, Mishra P, Sinha S, Raj S, Tripathi S (2021) FCS-fuzzy net: cluster head selection and routing-based weed classification in IoT with mapreduce framework. Wireless Netw 2021:1–19. https://doi.org/10.1007/S11276-021-02723-X
Sharma S, Verma Y, Vakhlu J (2021) Establishment of Agrobacterium rhizogenes-mediated hairy root transformation of Crocus sativus L. 3 Biotech 11(2):1–8. https://doi.org/10.1007/S13205-020-02626-2
Sinha S, Sandhu K, Bisht N, Naliwal T, Saini I, Kaushik P (2019) Ascertaining the paradigm of secondary metabolism enhancement through gene level modification in therapeutic plants. J Young Pharm 11(4):337–343. https://doi.org/10.5530/jyp.2019.11.70
Sinha S, Nailwal TK (2021) Quality assessment and evaluation of Oroxylum indicum through HPLC fingerprint and QAMS for important flavonoid components. Eurasian Chem Commun 3(1): 45–55. https://doi.org/10.22034/ECC.2021.121509
Sinha S, Nigam VK (2016) Poduction and characterization of L-glutaminase by Bacillus SP. Int J Pharm Sci Res 7(4): 1620–1626. https://doi.org/10.13040/IJPSR.0975-8232
Tiwari RK, Trivedi M, Guang ZC, Guo G-Q, Zheng G-C (2007) Genetic transformation of Gentiana macrophylla with Agrobacterium rhizogenes: growth and production of secoiridoid glucoside gentiopicroside in transformed hairy root cultures. Plant Cell Rep 26(2):199–210. https://doi.org/10.1007/s00299-006-0236-0
Author Information
Department of Biotechnology, Kumaun University, Nainital, India