Nutritional profiling and beneficial health attributes during developmental stages of an underutilized Spondias pinnata (L. F.) Kurz fruits for its optimum harvest
Research Articles | Published: 27 July, 2024
First Page: 1911
Last Page: 1922
Views: 2601
Keywords: Fruit developmental stages, Heath protective attributes, Nutritive value, S. pinnata (L. f.) Kurz.
Abstract
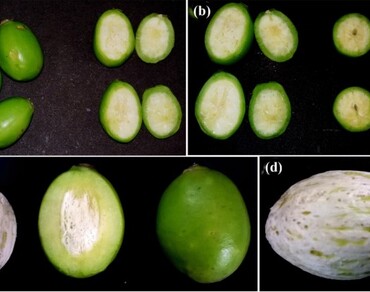
Finding novel food constituents that could benefit human beings in terms of nutritional and beneficial health attributes is an ever-expanding demand. In this direction, many underutilized traditional foods have been revisited. Spondias pinnata (L. f.) Kurz is an old-age native underutilized wild fruit plant used as either green or cooked vegetables in different recipes. The study aims to determine fruits nutritive and health-protective values in different developmental stages to focus on the critical stages for harvest and consumption. The nutritive value was determined by reducing sugar, total soluble solids (TSS), and phytochemical contents. The antioxidants were studied for DPPH, hydroxyl radical, and nitric oxide scavenging activity. It was observed that the highest ascorbic acid (0.28 mg ml−1) and a significant antioxidant activity (p < 0.05) were observed in the early (peel) stage with an IC50 value of 0.23 mg ml−1 (DPPH radical scavenging) and hydroxyl radical scavenging activity (IC50 value of 0.26 mg ml−1). The study revealed that the fruits have a good amount of nutrients and antioxidants that could substitute for present-day high-yielding fruit crops. Thus, it could be concluded that the fruits of S. pinnata have both nutritive and health-protective constituents in all stages that could contribute to human nutrition as a cheap and valuable food source.
References
Abaci ZT, Asma BM (2013) Examination of some physiological and biochemical changes based on ripening in fruits of different types of apricots. J Plant Sci 1(1):6–10
Augustyniak A, Bartosz G, Čipak A, Duburs G, Horáková L, Łuczaj W et al (2010) Natural and synthetic antioxidants: an updated overview. Free Radical Res 44(10):1216–1262. https://doi.org/10.3109/10715762.2010.508495
Baba SA, Malik SA (2015) Determination of total phenolic and flavonoid content, antimicrobial and antioxidant activity of a root extract of Arisaema jacquemontii Blume. J Taibah Univ Sci 9(4):449–454. https://doi.org/10.1016/j.jtusci.2014.11.001
Badoni A, Bisht C (2009) Importance and problems in natural regeneration of Spondias pinnata. Rep Opin 1(5):12–13
Bhuiyan M (2013) Pickle and chutney development from fresh Hog Plum (Spondias dulcis). J Environ Sci Nat Res 5(2):67–72. https://doi.org/10.3329/jesnr.v5i2.14604
Brito SA, Barbosa IS, de Almeida CLF, de Medeiros JW, Neto JCS, Rolim LA et al (2018a) Evaluation of gastroprotective and ulcer healing activities of yellow mombin juice from Spondias mombin L. PLoS ONE 13(11):e0201561. https://doi.org/10.1371/journal.pone.0201561
Brito SA, de Almeida CLF, de Santana TI, da Silva Oliveira AR, do Nascimento Figueiredo JCB, Souza IT et al (2018b) Antiulcer activity and potential mechanism of action of the leaves of Spondias mombin L. Oxid Med Cell Longev 2018:1–20. https://doi.org/10.1155/2018/1731459
Cabral B, Siqueira EMS, Bitencourt MAO, Lima MCJS, Lima AK, Ortmann CF et al (2016) Phytochemical study and anti-inflammatory and antioxidant potential of Spondias mombin leaves. Rev Bras 26(3):304–311. https://doi.org/10.1016/j.bjp.2016.02.002
Chaudhuri AB (1993) Forest plants of eastern India. APH Publishing, Delhi
Chaudhuri D, Ghate NB, Singh SS, Mandal N (2015) Methyl gallate isolated from Spondias pinnata exhibits anticancer activity against human glioblastoma by induction of apoptosis and sustained extracellular signal-regulated kinase 1/2 activation. Pharmacogn Mag 11(42):269–276. https://doi.org/10.4103/0973-1296.153078
Chaudhuri D, Ghate NB, Panja S, Mandal N (2016) Role of phenolics from Spondias pinnata bark in amelioration of iron overload-induced hepatic damage in Swiss albino mice. BMC Pharmacol Toxicol 17(1):1–14. https://doi.org/10.1186/s40360-016-0077-6
CI KC, Indira G (2016) Quantitative estimation of total phenolic, flavonoids, tannin and chlorophyll content of leaves of Strobilanthes Kunthiana (Neelakurinji). J Med Plants 4:282–286
da Silva ARA, de Morais SM, Mendes Marques MM, de Oliveira DF, Barros CC, de Almeida RR et al (2012) Chemical composition, antioxidant and antibacterial activities of two Spondias species from Northeastern Brazil. Pharm Biol 50(6):740–746. https://doi.org/10.3109/13880209.2011.627347
Das K, Roy D, Nandi P, Kundu S, Dutta P (2019) Spondias – an underutilized potential fruit crop of West Bengal. Acta Hort 1241:51–56
de Almeida CLF, Brito SA, de Santana TI, Costa HBA, de Carvalho Júnior CHR, da Silva MV et al (2017) Spondias purpurea L. (Anacardiaceae): antioxidant and antiulcer activities of the leaf hexane extract. Oxid Med Cell Longev 2017:1–14. https://doi.org/10.1155/2017/6593073
de Carvalho JM, Maia GA, da Fonseca AVV, de Sousa PHM, Rodrigues S (2015) Effect of processing on physicochemical composition, bioactive compounds and enzymatic activity of yellow mombin (Spondias mombin L.) tropical juice. J Food Sci Technol 52(2):1182–1187. https://doi.org/10.1007/s13197-013-1100-1
de Moura Barbosa H, Amaral D, do Nascimento JN, Machado DC, de Sousa Araújo TA, de Albuquerque UP et al (2018) Spondias tuberosa inner bark extract exert antidiabetic effects in streptozotocin-induced diabetic rats. J Ethnopharmacol 227:248–257. https://doi.org/10.1016/j.jep.2018.08.038
de Pinto G (2000) The composition of two Spondias gum exudates. Food Hydrocol. 14(3):259–263. https://doi.org/10.1016/S0268-005X(00)00005-9
de Sousa Araújo TA, de Almeida e Castro VTN, de Amorim ELC, de Albuquerque UP (2012) Habitat influence on antioxidant activity and tannin concentrations of Spondias tuberosa. Pharm Biol 50(6):754–759. https://doi.org/10.3109/13880209.2011.630673
Dubost J-M, Lamxay V, Krief S, Falshaw M, Manithip C, Deharo E (2019) From plant selection by elephants to human and veterinary pharmacopoeia of mahouts in Laos. J Ethnopharmacol 244:112157. https://doi.org/10.1016/j.jep.2019.112157
Floriano-Sánchez E, Floriano-Sánchez E, Villanueva C, Floriano-Sánchez E, Villanueva C, Medina-Campos ON et al (2006) Nordihydroguaiaretic acid is a potent in vitro scavenger of peroxynitrite, singlet oxygen, hydroxyl radical, superoxide anion and hypochlorous acid and prevents in vivo ozone-induced tyrosine nitration in lungs. Free Radical Res 40(5):523–533. https://doi.org/10.1080/10715760500419365
Foyer CH, Kyndt T, Hancock RD (2020) Vitamin C in Plants: Novel concepts, new perspectives, and outstanding issues. Antioxid Redox Signal 32(7):463–485. https://doi.org/10.1089/ars.2019.7819
Ghani A (1998) Medicinal plants of Bangladesh: chemical constituents and uses. Asiatic society of Bangladesh, Bangladesh
Gobinath R, Parasuraman S, Sreeramanan S, Enugutti B, Chinni SV (2022) Antidiabetic and antihyperlipidemic effects of methanolic extract of leaves of Spondias mombin in Streptozotocin-induced diabetic Rats. Front Physiol 13:870399. https://doi.org/10.3389/fphys.2022.870399
Hazra B, Biswas S, Mandal N (2008) Antioxidant and free radical scavenging activity of Spondias pinnata. BMC Complement Altern Med 8(1):63. https://doi.org/10.1186/1472-6882-8-63
Hernández Y, Lobo MG, González M (2006) Determination of vitamin C in tropical fruits: a comparative evaluation of methods. Food Chem 96(4):654–664. https://doi.org/10.1016/j.foodchem.2005.04.012
Iglesias DJ, Tadeo FR, Legaz F, Primo-Millo E, Talon M (2001) In vivo sucrose stimulation of colour change in citrus fruit epicarps: interactions between nutritional and hormonal signals. Physiol Plant 112(2):244–250. https://doi.org/10.1034/j.1399-3054.2001.1120213.x
Iqbal SS, Mujahid Md, Kashif SM, Khalid M, Badruddeen AM et al (2016) Protection of hepatotoxicity using Spondias pinnata by prevention of ethanol-induced oxidative stress, DNA-damage and altered biochemical markers in Wistar rats. Integ Med Res 5(4):267–275. https://doi.org/10.1016/j.imr.2016.05.002
Jannatizadeh A, Rezaei M, Rohani A, Lawson S, Fatahi R (2023) Towards modelling growth of apricot fruit: finding a proper growth model. Hortic Environ Biotechnol 64(2):209–222. https://doi.org/10.1007/s13580-022-00475-x
Khatoon S, Siddiqui HH, Hussain T, Firdous H (2015) Botanical and phytochemical evaluation of fruits of Spondias pinnata. Pharm Lett 7(5):276–280
Kumar S, Pandey AK (2013) Chemistry and biological activities of flavonoids: an overview. Sci World J 2013:1–16. https://doi.org/10.1155/2013/162750
Lin J-Y, Tang C-Y (2007) Determination of total phenolic and flavonoid contents in selected fruits and vegetables, as well as their stimulatory effects on mouse splenocyte proliferation. Food Chem 101(1):140–147. https://doi.org/10.1016/j.foodchem.2006.01.014
Lombardo VA, Osorio S, Borsani J, Lauxmann MA, Bustamante CA, Budde CO et al (2011) Metabolic profiling during peach fruit development and ripening reveals the metabolic networks that underpin each developmental stage. Plant Physiol 157(4):1696–1710. https://doi.org/10.1104/pp.111.186064
Lutsenko EA, Cárcamo JM, Golde DW (2002) Vitamin C prevents DNA mutation induced by oxidative stress. J Biol Chem 277(19):16895–16899. https://doi.org/10.1074/jbc.M201151200
Mandal V, Kundu S, Barman J, Adhikary R (2021) Harvesting strategy for different mango varieties based on comparative sugar and phenol contents. Proc Nat Acad Sci India Sect b: Biol Sci 91(1):1–11. https://doi.org/10.1007/s40011-020-01188-w
Mitchell JD, Daly DC (2015) A revision of Spondias L. (Anacardiaceae) in the Neotropics. PhytoKeys 55:1–92. https://doi.org/10.3897/phytokeys.55.8489
Mohammed M, Bridgemohan P, Graham O, Wickham L, Bridgemohan RSH, Mohammed Z (2019) Post-harvest physiology, biochemistry and quality management of Chili Plum (Spondias purpurea var. Lutea): a review. J Food Res 8(3):1. https://doi.org/10.5539/jfr.v8n3p1
Ognjanov V, Vujanić-Varga D, Mišić PD, Verešbaranji I, Macet K, Tešović Ž et al (1995) Anatomical and biochemical studies of fruit development in peach. Sci Hortic 64(1):33–48. https://doi.org/10.1016/0304-4238(95)00825-9
Prain D (1963) Bengal Plants, reprinted. Botanical Survey of India, Calcutta
Raghu V, Platel K, Srinivasan K (2007) Comparison of ascorbic acid content of Emblica officinalis fruits determined by different analytical methods. J Food Compos Anal 20(6):529–533. https://doi.org/10.1016/j.jfca.2007.02.006
Bora SN, Kakoti BB, Gogoi B, Goswami A (2014) Ethno-medicinal claims phytochemistry and pharmacology of Spondias pinnata: a review. Inte J Pharmaceut Sci Res 5:1138–1145
Sujarwo W, Keim AP (2021) Spondias pinnata (L.f.) Kurz. Anacardiaceae. In: Franco FM (ed) Ethnobotany of the Mountain Regions of Southeast Asia. Springer International Publishing, Cham, pp 1009–1014
Author Information
Plant and Microbial Physiology and Biochemistry Laboratory, Department of Botany, University of Gour Banga, Mokdumpur, India