Optimizing antioxidant potential and mitigating antinutritional factors in pearl millet (Pennisetum glaucum) via fermentation with Lactobacillus reuteri
Research Articles | Published: 23 October, 2024
First Page: 2425
Last Page: 2436
Views: 1682
Keywords: Fermentation, n Lactobacillus reuterin , Probiotic, Antioxidants, Antinutritional factors
Abstract
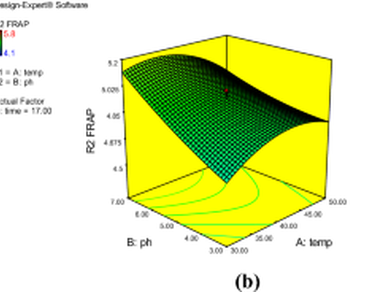
The study explores an innovative approach to enhance the nutritional value of Pearl Millet (Pennisetum glaucum) by harnessing the potential of Central Composite Design (CCD) for optimized fermentation with Lactobacillus reuteri. Fermentation by probiotics can improve nutrient quality, bioactive compounds, and reduce the antinutrients present in millet grains. Our research aims to enhance the antioxidant properties and simultaneously reduce antinutritional factors in Pearl Millet. Through meticulous CCD-driven fermentation, we strive to unlock the full potential of this valuable cereal crop, making it a more nutritionally potent and health-promoting food source. A research design with three variables, time of fermentation (4–30 h), fermentation temperature i.e. 30–50 °C, and pH ranging 3–7, was employed to investigate the effect on dependent variables, namely antioxidant activity, antioxidant content, and antinutritional factors. Analysis of the regression model showed significant correlation between the dependent variables and selected responses under consideration (p < 0.05). The most favourable parameters in the probiotic fermentation of pearl millet were obtained as a fermentation temperature of 30 ℃, a fermentation duration of 30 h, and a pH level of 7. Optimization showed a substantial enhancement in antioxidant activity and antioxidant content i.e. DPPH% 18.49%, FRAP 38%, ABTS 12.92%, TPC 14.84%, and TFC 34.86%. A significant reduction of 237% in tannin and 225% in phytic acid content was also observed.
References
Adebo OA, Medina-Meza IG (2020) Impact of fermentation on the phenolic compounds and antioxidant activity of whole cereal grains: a mini review. Molecules 25(4):927. https://doi.org/10.3390/molecules25040927
Baranyi J, Pin C, Ross T (1999) Validating and comparing predictive models. Int J Food Microbiol 48(3):159–166. https://doi.org/10.1016/s0168-1605(99)00035-5
Chinma CE, Ilowefah M, Muhammad K (2014) Optimization of rice bran fermentation conditions enhanced by baker’s yeast for extraction of protein concentrate. Niger Food J 32(1):126–132. https://doi.org/10.1016/s0189-7241(15)30105-3
De Ancos B, Sgroppo S, Plaza L, Cano MP (2002) Possible nutritional and health-related value promotion in orange juice preserved by high-pressure treatment. J Sci Food Agric 82(8):790–796. https://doi.org/10.1002/jsfa.1093
Duodu KG (2011). Effects of processing on antioxidant phenolics of cereal and legume grains. In Advances in cereal science: Implications to food processing and health promotion. American Chemical Society. pp. 31–54 https://doi.org/10.1021/bk-2011-1089.ch003
Elgailani IEH, Ishak CY (2014) Determination of tannins of three common Acacia species of Sudan. Adv Chem 2014:1–5. https://doi.org/10.1155/2014/192708
Elyas SH, El Tinay AH, Yousif NE, Elsheikh EA (2002) Effect of natural fermentation on nutritive value and in vitro protein digestibility of pearl millet. Food Chem 78(1):75–79. https://doi.org/10.1016/s0308-8146(01)00386-7
Jing L, Ma H, Fan P, Gao R, Jia Z (2015) Antioxidant potential, total phenolic and total flavonoid contents of Rhododendron anthopogonoides and its protective effect on hypoxia-induced injury in PC12 cells. BMC Complement Altern Med 15:1–12. https://doi.org/10.1186/s12906-015-0820-3
Karwowski MSM, de Andrade Cavalari CM, Oliveira K, Rosset M, de Macedo REF (2023) Millet Grains as an Immobilizing Matrix for Probiotics in Dry Fermented Sausage. Food and Bioprocess Technology. pp 1–13. https://doi.org/10.1007/s11947-023-03003-6
Kofuji K, Aoki A, Tsubaki K, Konishi M, Isobe T, Murata Y (2012) Antioxidant activity of β-glucan. Int Scholarly Res Notices. https://doi.org/10.5402/2012/125864
Lasekan O, Abbas K (2011) Investigation of the roasting conditions with minimal acrylamide generation in tropical almond (Terminalia catappa) nuts by response surface methodology. Food Chem 125(2):713–718. https://doi.org/10.1016/j.foodchem.2010.09.073
Mallick SA, Azaz K, Gupta M, Sharma V, Sinha BK (2013) Characterization of grain nutritional quality in wheat. Indian J Plant Physiol 18:183–186. https://doi.org/10.1007/s40502-013-0025-z
McFarland LV (2009) Evidence-based review of probiotics for antibiotic-associated diarrhea and Clostridium difficile infections. Anaerobe 15(6):274–280. https://doi.org/10.1016/j.anaerobe.2009.09.002
McKie VA, MccleAry BV (2016) A novel and rapid colorimetric method for measuring total phosphorus and phytic acid in foods and animal feeds. J AOAC Int 99(3):738–743. https://doi.org/10.5740/jaoacint.16-0029
Mutshinyani M, Mashau ME, Jideani AIO (2020) Bioactive compounds, antioxidant activity and consumer acceptability of porridges of finger millet (Eleusine coracana) flours: effects of spontaneous fermentation. Int J Food Prop 23(1):1692–1710. https://doi.org/10.1080/10942912.2020.1825485
Nambiar VS, Dhaduk JJ, Sareen N, Shahu T, Desai R (2011) Potential functional implications of pearl millet (Pennisetum glaucum) in health and disease. J Appl Pharm Sci. 2(7):62–67. https://doi.org/10.31989/ffhd.v2i7.85
Nkhata SG, Ayua E, Kamau EH, Shingiro JB (2018) Fermentation and germination improve nutritional value of cereals and legumes through activation of endogenous enzymes. Food Sci Nutr 6(8):2446–2458. https://doi.org/10.1002/fsn3.846
Rafiquzzaman SM, Kong IS, Kim JM (2015) Enhancement of antioxidant activity, total phenolic and flavonoid content of Saccharina japonica by submerged fermentation with Aspergillus oryzae. KSBB Journal 30(1):27–32. https://doi.org/10.7841/ksbbj.2015.30.1.27
Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C (1999) Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radical Biol Med 26(9–10):1231–1237. https://doi.org/10.1016/s0891-5849(98)00315-3
Saharan P, Sadh PK, Duhan JS (2017) Comparative assessment of effect of fermentation on phenolics, flavanoids and free radical scavenging activity of commonly used cereals. Biocatal Agric Biotechnol 12:236–240. https://doi.org/10.1016/j.bcab.2017.10.013
Salovaara H, Gänzle M (2011) Lactic acid bacteria in cereal-based products. Lactic Acid Bact Microbiol Funct Aspects 2:227. https://doi.org/10.1201/9780429057465-13
Sharma A, Kapoor AC (1996) Levels of antinutritional factors in pearl millet as affected by processing treatments and various types of fermentation. Plant Food Hum Nutr 49:241–252. https://doi.org/10.1007/bf01093221
Sibiya H, Bhagwat P, Amobonye A, Pillai S (2022) Effects of flaxseed and soybean supplementation on the nutritional and antioxidant properties of mahewu–a South African beverage. S Afr J Bot 150:275–284. https://doi.org/10.1016/j.sajb.2022.07.032
Singleton VL, Orthofer R, Lamuela-Raventós RM (1999) [14] Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Methods Enzymol 299:152–178. https://doi.org/10.1016/s0076-6879(99)99017-1
Sreeramulu D, Reddy C, Raghunath M (2009) Antioxidant activity of commonly consumed cereals, millets, pulses and legumes in India. Food Res Int. https://doi.org/10.1016/j.foodres.2009.10.006
Srivastava U, Saini P, Singh A (2020) Effect of natural fermentation on antioxidant activity of pearl millet (Pennisetum glaucum). Curr Nutr Food Sci 16(3):306–313. https://doi.org/10.2174/1573401314666181115103328
Srivastava U, Saini P, Singh A, Singh Z, Ahmed M, Iqbal U (2021) Enhancement in iron and folate by optimizing fermentation of barnyard millet by Lactobacillus plantarum using response surface methodology (RSM). Plant Arch. https://doi.org/10.2139/ssrn.4563200
Vaughn NA, Polnaszek C (2007) Design-Expert® software. Stat-Ease Inc, Minneapolis, p 55
Author Information
Centre of Food Technology, University of Allahabad, Prayagraj, India