Photoautotrophic micropropagation system (PAM): a novel approach deserving increased uptake for commercial plant production
Review Articles | Published: 27 January, 2021
First Page: 13
Last Page: 18
Views: 5063
Keywords: Micropropagation, Photoautotrophy, Photosynthesis, Ventilation, Automation
Abstract
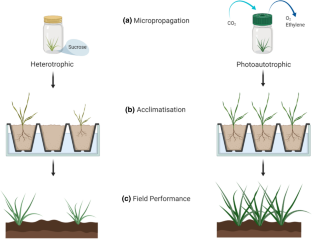
Most commercial plant tissue culture laboratories still use conventional micropropagation systems, requiring air-tight vessels and gelled medium supplemented with sugar. There is, however, a more efficient and economic system available, called “photoautotrophic micropropagation system” (PAM). Application of sugar-free medium and ventilated vessels are the key features of this system, which significantly improves plant physiological processes including photosynthesis and transpiration. The genetic basis for this has become more clear with a recent transcriptomic analysis of potato plantlets cultured on sugar-free medium showing the upregulation of more than 2000 genes, many of which are associated with photosynthesis efficiency and leaf cell development. The cultured plants from PAM exhibit improved vigour and health enabling a faster and smoother acclimatisation stage. Moreover, PAM is amenable to automation and scaling up by using large culture vessels/rooms. Here, we reviewed the recent studies on PAM and the issues/barriers to its commercialisation have also been discussed. Optimal culture conditions as well as different types of culture vessels are also explained.
References
- Afreen-Zobayed F, Zobayed SMA, Kubota C et al (2000) A combination of vermiculite and paper pulp supporting material for the photoautotrophic micropropagation of sweet potato. Plant Sci 157:225–231
- Ashloowalia BS, Prakash J, Savangikar VA (2002) Low cost options for tissue culture technology in developing countries. In: FAO/IAEA Technical Meeting
- Ashrafzadeh S (2020) In vitro grafting—twenty-first century’s technique for fruit tree propagation. Acta Agric Scand Sect B Soil Plant Sci 70:404–405
- Chen L, Lu Y, Hu Y, Xue X (2020) RNA-Seq sucrose-free medium improves the growth of potato (Solanum tuberosum L.) plantlets cultured in vitro. Plant Cell, Tissue Organ Cult 140:505–521
- Conner AJ (1987) Differential solasodine accumulation in photoautotrophic and heterotrophic tissue cultures of Solanum laciniatum. Phytochemistry 26:2749–2750
- Couceiro MA, Afreen F, Zobayed SMA, Kozai T (2006) Enhanced growth and quality of St. John’s wort (Hypericum perforatum L.) under photoautotrophic in vitro conditions. In Vitro Cell Dev Biol-Plant 42:278–282
- Cuenca B, Sánchez C, Aldrey A et al (2017) Micropropagation of axillary shoots of hybrid chestnut (Castanea sativa × C. crenata) in liquid medium in a continuous immersion system. Plant Cell Tissue Organ Cult 131:307–320
- Da Silva JAT, Giang DDT, Tanaka M (2006) Photoautotrophic micropropagation of Spathiphyllum. Photosynthetica 44:53–61
- Dubuc J-F, Desjardins Y (2004) Effects of autotrophic and mixotrophic tissue culture conditions on the expression of primary metabolism genes of tomato plantlets. In: II International symposium on acclimatization and establishment of micropropagated plants, vol 748, pp 165–171
- Fei L (2015) Towards automating micropropagation: from cells to shoots to plants in one step. Worcester Polytechnic Institute, Worcester
- Hoang NN, Kitaya Y, Morishita T et al (2017) A comparative study on growth and morphology of wasabi plantlets under the influence of the micro-environment in shoot and root zones during photoautotrophic and photomixotrophic micropropagation. Plant Cell Tissue Organ Cult 130:255–263
- Hoang NN, Kitaya Y, Shibuya T, Endo R (2020) Growth and physiological characteristics of wasabi plantlets cultured by photoautotrophic micropropagation at different temperatures. Plant Cell Tissue Organ Cult 143:1–10
- Iarema L, da Cruz ACF, Saldanha CW et al (2012) Photoautotrophic propagation of Brazilian ginseng [Pfaffia glomerata (Spreng.) Pedersen]. Plant Cell Tissue Organ Cult 110:227–238
- Ivanova M, Van Staden J (2010) Natural ventilation effectively reduces hyperhydricity in shoot cultures of Aloe polyphylla Schönland ex Pillans. Plant Growth Regul 60:143–150
- Khan PSSV, Kozai T, Nguyen QT et al (2002) Growth and net photosynthetic rates of Eucalyptus tereticornis Smith under photomixotrophic and various photoautotrophic micropropagation conditions. Plant Cell Tissue Organ Cult 71:141–146
- Kodym A, Leeb CJ (2019) Back to the roots: protocol for the photoautotrophic micropropagation of medicinal Cannabis. Plant Cell Tissue Organ Cult 138:399–402
- Kozai T, Koyama Y, Watanabe I (1988) Multiplication of potato plantlets in vitro with sugar free medium under high photosynthetic photon flux. In: Symposium on high technology in protected cultivation, vol 230, pp 121–128
- Kozai T (1991) Micropropagation under photoautotrophic conditions. Micropropagation. Springer, Berlin, pp 447–469
- Kozai T, Kubota C (2001) Developing a photoautotrophic micropropagation system for woody plants. J Plant Res 114:525–537
- Kozai T, Afreen F, Zobayed SMA (2005) Photoautotrophic (sugar-free medium) micropropagation as a new micropropagation and transplant production system. Springer Science and Business Media, Berlin
- Kozai T, Xiao Y (2008) A commercialized photoautotrophic micropropagation system. Plant tissue culture engineering. Springer, Berlin, pp 355–371
- Kozai T, Niu G, Takagaki M (2019) Plant factory: an indoor vertical farming system for efficient quality food production. Academic Press, New York
- Liao F, Wang B, Zhang M et al (2007) Response to sucrose-free culture and diffusive ventilation of plantlets in vitro of Gerbera jamesonii and photoautotrophic growth potential. Acta Hortic 764:257–264
- Mani M, Mathiyazhagan CR, Selvam P et al (2020) Foliar micro-morphology: a promising tool to improve survival percentage of tissue culture raised plantlets with special reference to in vitro propagation of Vitex negundo L. Vegetos 33:504–515
- Mazri MA, Meziani R, Elmaataoui S et al (2019) Assessment of genetic fidelity, biochemical and physiological characteristics of in vitro grown date palm cv. Al-Fayda Vegetos 32:333–344
- Park SY, Moon HK, Murthy HN, Kim YW (2011) Improved growth and acclimatization of somatic embryo-derived Oplopanax elatus plantlets by ventilated photoautotrophic culture. Biol Plant 55:559–562
- Pruski K, Astatkie T, Mirza M, Nowak J (2002) Photoautotrophic micropropagation of Russet Burbank potato. Plant Cell Tissue Organ Cult 69:197–200
- San José MC, Blázquez N, Cernadas MJ et al (2020) Temporary immersion systems to improve alder micropropagation. Plant Cell Tissue Organ Cult 143:1–11
- Sánchez C, Trasar C, Casalderrey M et al (2019) Micropropagation of willow shoots under photomixotrophic and photoautotrophic conditions: proliferation in liquid medium and acclimation in different soils. In: Conference of the IUFRO Working Party 2.09.02, pp 151–158
- Santos GC, Cardoso FP, Martins AD et al (2020) Effect of light and sucrose on photoautotrophic and photomixotrophic micropropagation of Physalis angulata. Biosci J 36:1353–1367
- Tanaka M, Giang DTT, Murakami A (2005) Application of a novel disposable film culture system to photoautotrophic micropropagation of Eucalyptus uro-grandis (Urophylia × grandis). In Vitro Cell Dev Biol-Plant 41:173–180
- Tisarum R, Samphumphung T, Theerawitaya C et al (2018) In vitro photoautotrophic acclimatization, direct transplantation and ex vitro adaptation of rubber tree (Hevea brasiliensis). Plant Cell Tissue Organ Cult 133:215–223
- Vidal N, Sánchez C (2019) Use of bioreactor systems in the propagation of forest trees. Eng Life Sci 19:896–915
- Wu H-C, Lin C-C (2013) Carbon dioxide enrichment during photoautotrophic micropropagation of Protea cynaroides L. plantlets improves in vitro growth, net photosynthetic rate, and acclimatization. HortScience 48:1293–1297
- Xiao Y, Kozai T (2004) Commercial application of a photoautotrophic micropropagation system using large vessels with forced ventilation: plantlet growth and production cost. HortScience 39:1387–1391
- Xiao Y, Niu G, Kozai T (2011) Development and application of photoautotrophic micropropagation plant system. Plant Cell Tissue Organ Cult 105:149–158
- Zia M, Yaqoob K, Mannan A et al (2020) Regeneration response of carnation cultivars in response of silver nanoparticles under in vitro conditions. Vegetos 33:11–20
- Zobayed SMA, Afreen F, Xiao Y, Kozai T (2004) Recent advancement in research on photoautotrophic micropropagation using large culture vessels with forced ventilation. In Vitro Cell Dev Biol-Plant 40:450–458
Author Information
Waimea Nurseries Ltd., Nelson, New Zealand