Photosynthetic efficiency and compositional alterations in microalgae Chlorella vulgaris in response to changes in the pH condition
Research Articles | Published: 16 February, 2021
First Page: 119
Last Page: 126
Views: 4299
Keywords: Chlorella vulgaris , pH effect, Photochemistry of PS II, Lipid production, Metabolic inhibitors
Abstract
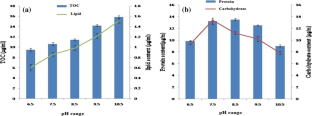
Present investigation on the effect of varying extracellular pH conditions (pH 6.5–10.5) on growth, accumulation of cell constituents and photosynthetic performance of microalga Chlorella vulgaris revealed that slightly alkaline pH (7.5–8.5) was the preferred pH range for growth, synthesis of protein and carbohydrate. Whereas total organic carbon (TOC) and lipid content in the microalga gradually increased with pH ranging ranging from 6.5 to 10.5. The FTIR results on total lipid (1740 cm− 1), lipid/carbohydrate (1740/1650 cm− 1) and lipid/protein (1740/1040 cm− 1) ratio in the cells grown at pH 6.5, 8.5 and 10.5 supported the above observation on the estimated amount of macromolecules. The chlorophyll fluorescence induction kinetics (OJIP), exhibiting various photosynthetic parameters, showed initial improvement in the photosynthetic efficiency between pH 6.5–8.5, followed by pH dependent declining pattern. Addition of HCO3− (20 mM) showed little effect on the photosynthetic electron transport. The energy dependent quenching parameters of chlorophyll fluorescence like ABS/RC, NPQ and qE, usually associated with the energy state of the membrane, registered an increasing trend with rising pH (pH 6.5–10.5). Use of energy inhibitors DNP, CCCP and electron acceptors/donors (methyl viologen and phenazine methosulphate) on ABS/RC, NPQ and qE suggested that pH dependent regulation of photosynthetic performance in C. vulgaris was tightly coupled with proton gradient of the membrane and that was reversed by the energy uncouplers like DNP and CCCP or electron acceptor like MV.
References
- Aslan S, Kapdan IK (2006) Batch kinetics of nitrogen and phosphorus removal from synthetic wastewater by algae. Ecol Eng 28:64–70
- Axelson L, Ryberg H, Beer S (1995) Two modes of bicarbonate utilization in the marine green macroalgae Ulva lactuca. Plant Cell Environ 18:439–445
- Azov Y (1982) Effect of pH on inorganic carbon uptake in algal cultures. Appl Environ Microbiol 43:1300–1306
- Bartley ML, Boeing WJ, Dungan BN, Holguin FO, Schaub T (2014) pH effects on growth and lipid accumulation of the biofuel microalgae Nannochloropsis salina and invading organisms. J Appl Phycol 26:1431–1437
- Bhattacharjee M (2016) Pharmaceutically valuable bioactive compounds of algae. Asian J Pharm Clin Res 7:43–47
- Chabrol E, Charonnat R (1973) Determination of total lipids. Press Med 45:1713–1720
- David JS, Ondrej P, Borowitzta MA (2011) Chlorophyll A fluorescence in aquatic science. Springer, Berlin
- Dean AP, Sigee DC, Estrada B, Pittman JK (2010) Using FTIR spectroscopy for rapid determination of lipid accumulation in response to nitrogen limitation in freshwater microalgae. Bioresour Technol 101:4499–4507
- Demmig-Adams B, Garab G, Adams William III, Govindgee (2014) Nonphotochemical quenching and energy dissipation in plants, algae and cyanobacteria, Advances in photosynthesis and respiration, vol 40. Springer Science + Business Media, Dordrecht
- Dubois M, Gilles KA, Hamilton JK, Rebers PA, Smith F (1956) Colorimetric method for determination of sugars and related substances. Anal Chem 28:350–356
- Edwards GE, Bovell CR (1996) Characteristics of a light-dependent proton transport in cells of Rhodospirillum rubrum. Biochimica et Biophysica Acta (BBA) Bioenerg 172:126–33
- Falkowski PG, Raven JA (2007) Photosynthesis and primary production in nature. Aquat Photosynth 319–63
- Frost-Christensen HK, Sand-Jensen (1990) Growth rate and carbon affinity of Ulva lactuca under controlled levels of carbon, pH and oxygen. Mar Biol 104:497–501
- Giordano M, Kansiz M, Heraud P, Beardall J, Wood B, Mcnaughton D (2001) Fourier Transform Infrared spectroscopy as a novel tool to investigate changes in intracellular macromolecular pools in the marine microalga Chaetoceros muellerii (Bacillariophyceae). J Phycol 37:271–279
- Goldman JC, Azov Y, Riley CB, Dennett MR (1982) The effect of pH in intensive microalgal cultures. I. Biomass regulation. J Exp Mar Biol Ecol 57:1–13
- Grobler DC, Davies E (1979) The use of the Walkley-Black method for organic carbon determination as a procedure for estimating algal yields. Water SA (Pretoria) 5:138–143
- Gupta N, Khare P, Singh DP (2019) Nitrogen-dependent metabolic regulation of lipid production in microalga Scenedesmus vacuolatus. Ecotoxicol Environ Saf 174:706–713
- Hofmann LC, Bischof K (2014) Ocean acidification effects on calcifying macroalgae. Aquat Biol 22:261–279
- Hohner R, Aboukila A, Kunz HH, Venema K (2016) Proton gradients and proton-dependent transport processes in the chloroplast. Front Plant Sci 29:7
- Juneja A, Ceballos RM, Murthy GS (2013) Effects of environmental factors and nutrient availability on the biochemical composition of algae for biofuels production: a review. Energies 6:4607–38
- Kosourov S, Seibert M, Ghirardi ML (2003) Effects of extracellular pH on the metabolic pathways in sulfur-deprived, H2-producing Chlamydomonas reinhardtii cultures. Plant Cell Physiol 45:146–155
- Krause GH, Weis E (1984) Chlorophyll fluorescence as a tool in plant physiology. II. Interpretation of fluorescence signals. Photosynth Res 5:139–157
- Li X, Li W, Zhai J, Wei H (2018) Effect of nitrogen limitation on biochemical composition and photosynthetic performance for fed-batch mixotrophic cultivation of microalga Spirulina platensis. Bioresour Technol 263:555–561
- Lichtenthaler HK, Buschmann C, Knapp M (2005) How to correctly determine the different chlorophyll fluorescence parameters and the chlorophyll fluorescence decrease ratio R Fd of leaves with the PAM fluorometer. Photosynthetica 43:379–393
- Lichtenthaler HK, Rinderle U (1988) The role of chlorophyll fluorescence in the detection of stress conditions in plants. CRC Crit Rev Anal Chem 1:29–85
- Lignell A, Pederson M (1989) Agar composition as a function of morphology and growth rate on some morphological strains of Gracilaria secundata and Gracilaria verrucosa (Rhodophyta). Bot Mar 32:219–227
- Logan BA, Adams WW III, Demming-Adams (2007) Avoiding common pitfalls of chlorophyll fluorescence analysis under field conditions. Funet Plant Biol 34:853–859
- Lowry OH, Farr AL, Randall RJ, Rosebrough NJ (1951) Protein measurement with the folin phenol reagent. J Biol Chem 193:265–275
- Lu X, Zhang Z, Xu Y, Lu J, Tang W, Zhang J (2020) Effect of new carbonyl cyanide aromatic hydrazones on biofilm inhibition against methicillin resistant Staphylococcus aureus. RSC Adv 10(30):17854–61
- Masojıdek J, Koblızek M, Torzillo G (2004) Photosynthesis in microalgae. In: Handbook of microalgal culture: biotechnology and applied phycology, vol 20
- Moheimani NR, Borowitzka MA (2011) Increased CO 2 and the effect of pH on growth and calcification of Pleurochrysis carterae and Emiliania huxleyi (Haptophyta) in semi continuous cultures. Appl Microbiol Biotechnol 90:1399–1407
- Moreland DE (1980) Mechanisms of action of herbicides. Annu Rev Plant Physiol 31:597–638
- Morris I, Glover H, Yentsch C (1974) Products of photosynthesis by marine phytoplankton: the effect of environmental factors on the relative rates of protein synthesis. Mar Biol 27:1–9
- Mosley LM, Peake BM, Hunter KA (2010) Modelling of pH and inorganic carbon speciation in estuaries using the composition of the river and seawater end members. Environ Model Softw 25(12):1658–1663
- Mus F, Cournac L, Cardettini V, Caruana A, Peltier G (2005) Inhibitor studies on non-photochemical plastoquinone reduction and H2 photoproduction in Chlamydomonas reinhardtii. Biochimica et Biophysica Acta (BBA) Bioenerg 1708:322–332
- Norici A, Bazzoni AM, Pugnetti A, Raven JA, Giordano M (2011) Impact of irradiance on the C allocation in the coastal marine diatom Skeletonema marinoi Sarno and Zingone. Plant Cell Environ 34:1666–1677
- Perkins RG, Mouget J-L, Lefebvre S, Lavaud J (2006) Light response curve methodology and possible implications in the application of chlorophyll fluorescence to benthic diatoms. Mar Biol 149:703–712
- Pistorius AM, DeGrip WJ, Egorova-Zachernyuk TA (2009) Monitoring of biomass composition from microbiological sources by means of FT‐IR spectroscopy. Biotechnol Bioeng 103:123–129
- Qi M, Yao C, Sun B, Cao X, Fei Q, Liang B, Ran W, Xiang Q, Zhang Y, Lan X (2019) Application of an in situ CO2–bicarbonate system under nitrogen depletion to improve photosynthetic biomass and starch production and regulate amylose accumulation in a marine green microalga Tetraselmis subcordiformis. Biotechnol Biofuels 12:184
- Richmond A, Becker EW (1986) Technological aspects of mass cultivation—a general outline. In: Handbook of microalgal mass culture, pp 245–264
- Schreiber U (2004) Pulse-amplitude-modulation (PAM) fluorometry and saturation pulse method: an overview. In: Chlorophyll a fluorescence. Springer, Dordrecht, pp 279–319
- Singh SP, Singh P (2014) Effect of CO2 concentration on algal growth: a review. Renew Sustain Energy Rev 38:172–179
- Slooten L, Branders C (1979) The influence of energy-transfer inhibitors on proton permeability and photophosphorylation in normal and preilluminated Rhodospirillum rubrum chromatophores. Biochimica et Biophysica Acta BBA Bioenerg 547:79–90
- Smith FA, Raven JA (1979) Intracellular pH and its regulation. Annu Rev Plant Physiol 30:289–311
- Strasser RJ, Srivastava A, Tsimilli-Michael M (2000) The fluorescence transient as a tool to characterize and screen photosynthetic samples. In: Yunus M, Pathre U, Mohanty P (eds) Probing photosynthesis: mechanism, regulation and adaptation, Chapter 25. Taylor and Francis, London, pp 445–483
- Taraldsvik M, Myklestad SM (2000) The effect of pH on growth rate, biochemical composition and extracellular carbohydrate production of the marine diatom Skeletonema costatum. Eur J Phycol 35:189–194
- Vadlamani A, Viamajala S, Pendyala B, Varanasi S (2017) Cultivation of microalgae at extreme alkaline pH conditions: a novel approach for biofuel production. ACS Sustain Chem Eng 5:7284–7294
- Van Wagenen J, Miller TW, Hobbs S, Hook P, Crowe B, Huesemann M (2012) Effects of light and temperature on fatty acid production in Nannochloropsis salina. Energies 5:731–740
- Xiang R, Shi J, Zhang H, Dong C, Liu L, Fu J, He X, Yan Y, Wu Z (2018) Chlorophyll a fluorescence and transcriptome reveal the toxicological effects of bis-phenol A on an invasive cyanobacterium, Cylindrospermopsis raciborskii. Aquat Toxicol 200:188–196
- Yang Y, Kadim MI, Khoo WJ, Zheng Q, Setyawati MI, Shin YJ, Lee SC, Yuk HG (2014) Membrane lipid composition and stress/virulence related gene expression of Salmonella enteritidis cells adapted to lactic acid and trisodium phosphate and their resistance to lethal heat and acid stress. Int J Food Microbiol 191:24–31
- Yu KL, Show PL, Ong HC, Ling TC, Lan JC, Chen WH, Chang JS (2017) Microalgae from wastewater treatment to biochar feedstock preparation and conversion technologies. Energy Convers Manag 150:1–3
- Zhang Z, Geng L, Huiyuan G, Litao Y, Cheng Y, Peng L, Qingwei M (2012) Characterization of photosynthetic performance during senescence in stay-green and quick-leaf-senescence Zea mays L. Inbred Lines. PLOS/ONE. https://doi.org/10.1371/journal.pone.0042936
Author Information
Department of Environmental Science, Babasaheb Bhimrao Ambedkar University, Lucknow, India