Physiological and gene expression studies of selected Zea mays L. and Pennisetum glaucum (L.) R. Br. genotypes to simulated drought stress condition
Research Articles | Published: 14 June, 2019
First Page: 397
Last Page: 406
Views: 3844
Keywords: Chlorophyll fluorescence, Drought stress, Gene expression, Reactive oxygen species, Pennisetum glaucum , Zea mays
Abstract
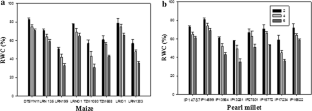
Drought is a major environmental stress that significantly obstructs productivity of various crops worldwide. Among several remedial methods to conquer this abiotic stress, use of resistant cultivars is most preferred. This study compares the response in selected genotypes of pearl millet and maize under a progressive drought stress conditions at specific intervals. Three genotypes (IP14599, IP14787 and LRNO3) of pearl millet recorded relatively higher water content (RWC) than the three genotypes (DTSYN11, LRN03 and LRIO1) of maize. Leaf water potential (ΨL), leaf osmotic potential (Ψπ), leaf turgor potential (Ψp) and PSII were measured in harvests to attain comparative observations. Furthermore, to correlate these results expression of three genes were measured. It was observed that Ψπ decreased over time and Ψp recorded a decrease with ΨLat a higher rate in maize compared to pearl millet. A more declining trend in maximum fluorescence (∆F/Fm′) and electron transport rate (ETR) in LRIOI, LRNO1 and DTSYN11 was recorded compared to IP14599, IP14787 and LRNO3. The study of gene CBF in leaves and roots revealed it’s responsiveness to drought in genotypes of pearl millet IP14599, LRNO3 and IP14787 while it was absent in maize genotypes LRNO1 and LRIO1, with the exception of DTSYN11. Expression of RubSc gene showed a noteworthy decline in reactive oxygen species in the genotypes IP14599, LRNO3, DTSYN11 and IP14787, while a marked increase was observed in LRNO1 and LRIO1. Likewise gene PIP2;3 were highly responsive to drought in pearl millet but not in maize, where they might support greater water transport. Overall, the results indicate remarkable activation of mRNA expression of these genes under drought stress which provides the resistance against drought.
References
- Bartels D, Sunkar R (2005) Drought and salt tolerance in plants. Crit Rev Plant Sci 24:23–58
- Bray EA, Bailey-Serres J, Weretilnyk E (2000) Responses to abiotic stresses. In: Buchanan BB, Gruissem W, Jones R (eds) Biochemistry and molecular biology of plants. American Society of Plant Physiologists, Rockville, pp 1158–1203
- Bruce WB, Edmeades GO, Barker TC (2002) Molecular and physiological approaches to maize improvement for drought tolerance. J Exp Bot 53(366):13–25
- Chaves MM, Maroco JP, Pereira JS (2002) Understanding plant responses to drought—from genes to the wholeplant. Funct Plant Biol 30:239–264. https://doi.org/10.1071/FP02076
- Davies WJ, Zhang JH (1991) Root signals and the regulation of growth and development of plants in drying soil. Annu Rev Plant Physiol Plant Mol Biol 42:55–76
- Degenkolbe T, Do P, Zuther E, Repsilber D, Walther D (2009) Expression profiling of rice cultivars differing in their tolerance to long-term drought stress. Plant Mol Biol 69:133–153
- Farooq M, Hussain M, Wahid A, Siddique KHM (2012) Drought stress in plants: an overview. In: Aroca R (ed) Plant responses to drought stress: from morphological to molecular features. Springer, Berlin, pp 1–37
- Farre I, Van Oijen M, Leffelaar PA, Faci JM (2000) Analysis of maize growth for different irrigation strategies in northeastern Spain. Eur J Agron 12:225–238
- Feng Q, Masayuki K, Yoh S (2014) Genome wide identification of housekeeping gene in maize. Plant Mol Biol 86:543–554
- Fracasso A, Luisa T, Stefano A (2016) Drought tolerance strategies highlighted by two Sorghum bicolor races in a dry-down experiment. J Plant Physiol 190:1–14
- Frova C, Krajewski P, Villa M, Sari-Gorla M (1999) Genetic analysis for drought tolerance in stress and non-drought stress environments. Afr Crop Sci J 10(1):1–9
- Genty B, Briantails J, Baker NR (1989) The relationship between the quenching yield of photosynthetic electron transport rate and quenching of chlorophyll fluorescence. Biochem Biophys Acta 990:87–92
- Hassan SA, Rabei SH, Nada RM, Abogadallah GM (2017) Water use efficiency in the drought-stressed sorghum and maize in relation to expression of aquaporin genes. Biol Plant 61:127–137
- Hayano-Kanashiro C, Calderon C, Ibarra-Laclette E, Herrera-Estrella L, Simpson J (2009) Analysis of gene expression and physiological responses in three mexican maize landraces under drought stress and recovery irrigation. PLoS One 4(10):e7531. https://doi.org/10.1371/journal.pone.0007531
- The International Institute of Tropical Agriculture (IITA) (2014) Nigerian’s maize industry: statistical handbook
- Iwuala EN, Odjegba V, Sharma V, Umebese C, Alam A (2018) Differential traits of two genotypes of Pennisetum glaucum (L.) R. Br. under simulated drought conditions. Vegetos Int J Plant Res 31(Special):115–120
- Jaleel CA, Gopi R, Panneerselvam R (2007) Alterations in lipid peroxidation, electrolyte leakage andproline metabolism in Catharanthus roseus under treatment with triadimefon, a systemic fungicide. Comptes Rendus Biol 330(12):905–912
- Jaleel CA, Gopi R, Sankar B, Gomathinayagam M, Panneerselvam R (2008) Differential responses in water use efficiency in two varieties of Catharanthus roseus under drought stress. Comptes Rendus Biol 331:42–47
- Jones MM, Rawson HM (1979) Influence of rate of development of leaf water deficits upon photosynthesis, leaf conductance, water use efficiency and osmotic potential in sorghum. Plant Physiol 45:103–111
- Lambers H, Chapin FS III, Pons T (2008) Plant physiological ecology. Springer, New York, pp 163–223
- Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25:402–408
- Massacci A, Nabiev SM, Pietrosant L, Thor K, Liepner J (2008) Response of the photosynthetic apparatus of cotton (Gossypium hirsutum) to the onset of drought stress under field conditions studied by gas exchange analysis and chlorophyll fluorescence imaging. Plant Physiol Biochem 46(2):189–195
- Murata N, Takahashi S, Nishiyama Y, Allakhverdiev SI (2007) Photoinhibition of photosystem 11 under environmental stress. Biochem Biophys Acta 176:414–421
- Nawaz K, Hussain K, Majeed A, Khan F, Afghan S, Ali K (2010) Fatality of salt stress to plants: morphological, physiological and biochemical aspects. Afr J Biotech 9(34):5475–5480
- Nogues S, Alegre L, Araus J, Perez-Aranda L, Lannoye R (1994) Modulated chlorophyll fluorescence and photosynthetic gas exchange as rapid screening methods for drought tolerance in barley genotypes. Photosynthetica 30:465–474
- Ort DR (2001) when there is too much light. Plant Physiol 125:29–32
- Pannu RK, Singh DP (1993) Effect of irrigation on soil plant water relations and canopy photosynthesis in mungbean (Vigna radiata) (L). Trop Agric Trinidad 70:153–161
- Razmjoo K, Heydarizadeh P, Sabzalian MR (2008) Effect of salinity and drought stresses on growth parameters and essential oil content of Matricaria chamomile. Int J Agric Biol 10:451–454
- Reddy PS, Srinivas D, Kiran K, Bhatnagar-Mathur Vadez V (2015) Cloning and validation of reference genes for normalization of gene expression studies in pearl millet (Pennisetum glaucum (L.) R. Br.) by quantitative real-time PCR. Plant Gene 1:35–42
- Schreiber U, Bilger W, Neubauer C (1995) Chlorophyll fluorescence as a nonintrusive indicator for rapid assessment for in vitro photosynthesis. In: Schulze ED, Cadwell MM (eds) Ecophysiology of photosynthesis. Springer, Berlin, pp 49–70
- Sharma V, Kumari N, Gheek N (2014) Drought-induced changes in chlorophyll fluorescence, photosynthetic pigments, and thylakoid membrane proteins of Vigna radiata. J Plant Interact 9(1):712–721
- Shinozaki K, Yamaguchi-Shinozaki K, Seki M (2007) Regulatory network of gene expression in the drought and cold stress responses. Curr Opin Plant Biol 6:410–417
- Singh BR, Singh DP (1995) Agronomic and physiological response of sorghum, maize and pearl millet to irrigation. Field Crop Res 42:57–67
- Tuinstra MR, Grote EM, Goldsbrough PB, Ejeta G (1997) Genetic analysis of post flowering drought tolerance and components of grain development in Sorghum bicolor(L.) Moench. Mol Breed 3:439–448
- Turner NC (1981) Techniques and experimental approaches for the measurement of plant water status. Plant Soil 58:339–366
- Xue P, McIntyre C, Glassop D, Shorter R (2008) Use of expression analysis to dissect alterations in carbohydrate metabolism in wheat leaves during drought stress. Plant Mol Biol 67:197–214
- Yadav OP, Rai KN, Gupta SK (2012) Pearl millet: genetic improvement for tolerance to abiotic stress. In: Tuteja N, Gill SS, Tuteja R (eds) Improving crop productivity in sustainable agriculture. Wiley-VCH Verlag GmbH & Co. KGaA, New York, pp 261–288
Author Information
Department of Plant Science and Biotechnology, Federal University Oye Ekiti, Ekiti, Nigeria