Phytochemical analysis of various medicinal plants and their activity against ESKAPE strains
*Article not assigned to an issue yet
Research Articles | Published: 13 December, 2024
First Page: 0
Last Page: 0
Views: 2420
Keywords: Phytochemicals, Plant extracts, Bioactivities, Health benefits, Antioxidants, Natural compounds
Abstract
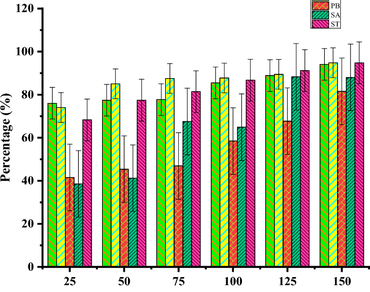
Medicinal plants are an important resource in traditional healthcare systems, especially in regions with limited access to modern medicine. With the growing need for alternative therapeutic agents in the fight against antibiotic-resistant pathogens, medicinal plants offer a promising source of bioactive compounds. This study aims to investigate the phytochemical composition and antimicrobial activity of five medicinal plants commonly used in traditional medicine—Acalypha indica, Hibiscus rosa sinensis, Piper betel, Senna auriculata, and Solanum trilobatum against ESKAPE pathogens (Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa, and Enterobacter species). Organic solvent extraction was performed using a Soxhlet apparatus with acetone, methanol, and ethanol as solvents. Phytochemical analysis revealed the presence of proteins, carbohydrates, phenols, and tannins, flavonoids, and saponins in all examined plants. The results demonstrate that both aqueous and organic solvent extracts of these plants contain bioactive compounds with potential therapeutic applications, supporting their traditional use in treating various diseases. The findings reinforce the conventional use of these plants for treating infectious diseases and emphasize the need for continued exploration of plant-based therapeutics in modern medicine.
References
Arulkumar A, Rosemary T, Paramasivam S, Rajendran RB (2018) Phytochemical composition, in vitro antioxidant, antibacterial potential and GC-MS analysis of red seaweeds (Gracilaria corticata and Gracilaria edulis) from Palk Bay. Biocat Agricult Biotech, India. https://doi.org/10.1016/j.bcab.2018.05.008
Bhakshu LM, Ratnam KV, Raju RV (2023) An Insight of phytochemical and pharmacological prospective of senna auriculata (L) Roxb. Bioact Pharmacol Legumes. https://doi.org/10.1201/9781003304555
Bhalodia NR, Shukla VJ (2011) Antibacterial and antifungal activities from leaf extracts of Cassia fistula l.: an ethnomedicinal plant. J Adv Pharmaceut Technol Res. https://doi.org/10.4103/2231-4040.82956
Bibi Y, Nisa S, Chaudhary FM, Zia M (2011) Antibacterial activity of some selected medicinal plants of Pakistan. BMC Complem Altern Med. https://doi.org/10.1186/1472-6882-11-52
Bordean ME, Ungur RA, Toc DA, Borda IM, Marțiș GS, Pop CR, Muste S (2023) Antibacterial and phytochemical screening of Artemisia species. Antioxidants 12(3):596. https://doi.org/10.3390/antiox12030596
Brahma P, Baruah S (2023) Investigation of phytochemical constituents GC-MS DPPH free radical scavenging assay and mineral contents of Glochidion sphaerogynum Mull Arg Kurz bark extract. Plant Sci Today. https://doi.org/10.14719/pst.2019
Brinda P, Sasikala P, Purushothaman KK (1981) Pharmacognostic studies on Merugan kizhangu. Bull Med Eth Bot Res 3(1):84–96
Devi NN, Singh MS (2013) GC-MS Analysis of metabolites from endophytic fungus Colletotrichum gloeosporioides isolated from Phlogacanthus thyrsiflorus Nees. Int J Pharm Sci 23(2):392–395
Dhanani T, Shah S, Gajbhiye NA, Kumar S (2017) Effect of extraction methods on yield, phytochemical constituents, and antioxidant activity of Withania somnifera. Arab J Chem. https://doi.org/10.1016/j.arabjc.2013.02.015
Doddanna SJ, Patel S, Sundarrao MA, Veerabhadrappa RS (2013) Antimicrobial activity of plant extracts on Candida albicans: an: in vitro: study. Indian J Dent Res 24(4):401–405. https://doi.org/10.4103/0970-9290.118358
Duarte GV, Ramarao BV, Amidon TE, Ferreira PT (2011) Effect of hot water extraction on hardwood kraft pulp fibers (Acer saccharum, sugar maple). Ind Eng Chem Res 50(17):9949–9959. https://doi.org/10.1021/ie200639u
Dubale S, Kebebe D, Zeynudin A, Abdissa N, Suleman S (2023) Phytochemical screening and antimicrobial activity evaluation of selected medicinal plants in Ethiopia. J Exp Pharmacol. https://doi.org/10.2147/JEP.S379805
Dzięcioł M, Wróblewska A, Janda-Milczarek K (2023) Comparative studies of DPPH radical scavenging activity and content of bioactive compounds in maca (Lepidium meyenii) root extracts obtained by various techniques. Appl Sci 13(8):4827. https://doi.org/10.3390/app13084827
Dzotam JK, Touani FK, Kuete V (2015) Antibacterial and antibiotic-modifying activities of three food plants (Xanthosoma mafaffa Lam Moringa oleifera (L) Schott and Passiflora edulis Sims) against multidrug-resistant (MDR) Gram-negative bacteria. BMC Complement Altern Med. https://doi.org/10.1186/s12906-016-0990-7
Efenberger-Szmechtyk M, Nowak A, Czyzowska A (2021) Plant extracts rich in polyphenols: Antibacterial agents and natural preservatives for meat and meat products. Crit Rev Food Sci Nutr 61(1):149–178. https://doi.org/10.1080/10408398.2020.1722060
Gomare KS, Mishra DN (2018) FTIR spectroscopic analysis of phytochemical extracts from Hibiscus rosa–sinensis L. used for hair disorder. Intern J Recent Trends Sci Technol.70–75.
Gupta A, Jeyakumar E, Lawrence R (2021) Journey of limonene as an antimicrobial agent. J Pure Appl Microbio. https://doi.org/10.22207/JPAM.15.3.01
Hemeg HA, Moussa IM, Ibrahim S, Dawoud TM, Alhaji JH, Mubarak AS, Marouf SA (2020) Antimicrobial effect of different herbal plant extracts against different microbial populations. Saudi J Biolog Sci 27(12):3221–3227. https://doi.org/10.1016/j.sjbs.2020.08.015
Huang J, Zhan G, Zheng B, Sun D, Lu F, Lin Y, Li Q (2011) Biogenic silver nanoparticles by Cacumen platycladi extract: synthesis, formation mechanism, and antibacterial activity. Ind Eng Chem Res 50(15):9095–9106. https://doi.org/10.1021/ie200858y
Khalid S, Arshad M, Mahmood S et al (2023) Extraction and quantification of moringa oleifera leaf powder extracts by HPLC and FTIR. Food Anal Methods. https://doi.org/10.1007/s12161-023-02470-z
Khan AA, Shad SA, Akram W (2013a) Resistance to new chemical insecticides in the house fly Musca domestica L. from dairies in Punjab, Pakistan. Parasitol Res. https://doi.org/10.1007/s00436-013-3365-8
Khan UA, Rahman H, Niaz Z, Qasim M, Khan J, Tayyaba, et al (2013b) Antibacterial activity of some medicinal plants against selected human pathogenic bacteria. Eur J Microbiol Immunol. https://doi.org/10.1556/eujmi.3.2013.4.6
Kirtikar KR, Basu BD, Blatter E (1975) Indian medicinal plants, periodical experts. Delhi 2:999
Kumar VP, Chauhan NS, Padh H, Rajani M (2006) Search for antibacterial and antifungal agents from selected Indian medicinal plants. J Ethnopharmacol 107(2):182–188. https://doi.org/10.1016/j.jep.2006.03.013
Kumar S, Ratha KK, Jaiswal S, Rao MM, Acharya R (2024) Exploring the potential of Andrographis paniculata and its bioactive compounds in the management of liver diseases: A comprehensive food chemistry perspective. Food Chem Advan. https://doi.org/10.1016/j.focha.2024.100674
Lu H, Hu R, Ward A, Amidon TE, Liang B, Liu S (2012) Hot-water extraction and its effect on soda pulping of aspen woodchips. Biomass Bioenerg 39:5–13. https://doi.org/10.1016/j.biombioe.2011.01.054
Mishra MK, Pandey S, Niranjan A, Misra P (2021) Comparative analysis of phenolic compounds from wild and in vitro propagated plant Thalictrum foliolosum and antioxidant activity of various crude extracts. Chem Papers. https://doi.org/10.1007/s11696-021-01708-6
Mujeeb F, Bajpai P, Pathak N (2014) Phytochemical evaluation, antimicrobial activity, and determination of bioactive components from leaves of Aegle marmelos. Biomed Res Int. https://doi.org/10.1155/2014/497606
Naskar S, Mazumder UK, Pramanik G, Bala A, Haldar PK, Islam A, Gupta M (2011) Comparative in vitro antioxidant activity of different parts of Cocos nucifera (Linn.) on reactive oxygen and nitrogen species. Int j Pharm Pharm Sci 3(3):104–107
Samaraweera T, Samaraweera T, Senadeera N, Ranaweera CB (2023) Evaluation of antibacterial activity of endemic jeffreycia zeylanica plant found in Sri Lanka. South Asian J Res Microbiol 16(1):1–9. https://doi.org/10.9734/sajrm/2023/v16i1296
SubbuThavamurugan DM, Suresh P et al (2023) Investigation on nutritional, phytochemical, and antioxidant abilities of various traditional rice varieties. Appl Biochem Biotechnol 195:2719–2742. https://doi.org/10.1007/s12010-022-04264-1
Talib WH, Mahasneh AM (2010) Antimicrobial, cytotoxicity, and phytochemical screening of Jordanian plants used in traditional medicine. Molecules 15(3):1811–1824. https://doi.org/10.3390/molecules15031811
Xu GH, Chen JC, Liu DH, Zhang YH, Jiang P, Ye XQ (2008) Minerals, phenolic compounds, and antioxidant capacity of citrus peel extract by hot water. J Food Sci 73(1):11–18. https://doi.org/10.1111/j.1750-3841.2007.00546.x
Yasmin F, Nazli IH, Shafiq N, Aslam M, Bin Jardan YA, Nafidi HA, Bourhia M (2023) Plant-based bioactive phthalates derived from Hibiscus rosa-sinensis: In vitro and silico enzyme inhibition. ACS Omega 8(36):32677–32689. https://doi.org/10.1021/acsomega.3c03342
Author Information
Rhizosphere Biology Laboratory, Department of Microbiology, Bharathidasan University, Trichy, India