Phytochemical profiling using FTIR- and LC-HRMS-based metabolomics, mineral content, and free radical scavenging activity of Sonchus arvensis extracts from different growth locations
*Article not assigned to an issue yet
Research Articles | Published: 30 January, 2025
First Page: 0
Last Page: 0
Views: 2052
Keywords: Antioxidants, Minerals, Metabolite profiling, n Sonchus arvensisn , Metabolomics
Abstract
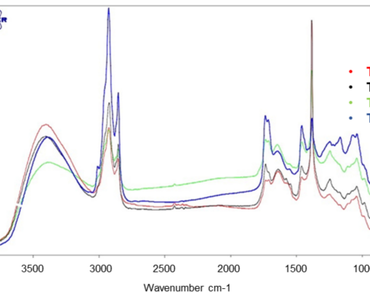
Sonchus arvensis is a medicinal plant widely used to treat hypertension, kidney stones, and gout. This work aims to profile the metabolites, minerals content, and free radical scavenging activity of S. arvensis from different growth locations. We analyzed total phenolics content (Folin-Ciocalteu method), free radical scavenging activity (DPPH method), levels of eight flavonoid compounds (HPLC), and minerals content (ICP-OES). We also performed FTIR fingerprint analysis and UHPLC-Q-Orbitrap HRMS metabolite profiling. S. arvensis from Malang gave the highest extraction yield, total phenolic content, levels of eight flavonoid compounds, free radical scavenging activity, and the highest metabolite content compared with S. arvensis from other regions used in this study. K and Ca are minerals with the highest content in S. arvensis extract, while B, Cu, and Zn have concentrations < 100 mg/kg. The FTIR spectra of all S. arvensis had a similar pattern, indicating that the extracted metabolites were not significantly different except for the levels of the compounds. Metabolite profiling using UHPLC-Q-Orbitrap HRMS successfully identified 36 compounds, most belonging to the phenolic acid and flavonoid groups. Distinguishing S. arvensis from different growth locations was performed using principal component analysis (PCA) with the FTIR spectra’ absorbance and hierarchical cluster analysis (HCA) of the identified compounds’ peak areas as the variables. PCA was able to classify each sample based on the growth location. Differences in the metabolite profiles were observed in S. arvensis from different growth locations, which affected its free radical scavenging activities.
References
Aziz Z, Yuliana ND, Simanjuntak P, Rafi M, Mulatsari E, Abdillah S (2021) Investigation of yacon leaves (Smallanthus Sonchifolius) for α-glucosidase inhibitors using metabolomics and in silico approach. Plant Foods Hum Nutr 76:487–493. https://doi.org/10.1007/s11130-021-00926-3
Bai Y, Zheng Y, Pang W, Peng W, Wu H, Yao H, Li P, Deng W, Cheng J, Su W (2018) Identification and comparison of constituents of aurantii fructus and aurantii fructus immaturus by UFLC-DAD-Triple TOF-MS/MS. Molecules 23:803. https://doi.org/10.3390/molecules23040803
Berton SBR, Cabral MRP, de Jesus GAM, Sarragiotto MH, Pilau EJ, Martins AF, Bonafé EG, Matsushita M (2020) Ultra-high-performance liquid chromatography supports a new reaction mechanism between free radicals and ferulic acid with antimicrobial and antioxidant activities. Ind Crops Prod 154:112701. https://doi.org/10.1016/j.indcrop.2020.112701
Borges TH, López LC, Pereira JA, Cabrera–Vique C, Seiquer I (2017) Comparative analysis of minor bioactive constituents (CoQ 10, tocopherols and phenolic compounds) in Arbequina extra virgin olive oils from Brazil and Spain. J Food Compost Anal 63:47–54. https://doi.org/10.1016/j.jfca.2017.07.036
Bunaciu AA, Aboul-Enein HY, Fleschin S (2011) Recent applications of fourier transform infrared spectrophotometry in herbal medicines analysis. Appl Spectrosc Rev 46:251–260
Das S, Majumder T, Sarkar A, Mukherjee P, Basu S (2020) Flavonoids as BACE1 inhibitors: QSAR modelling, screening and in vitro evaluation. Int J Biol Macromol 165:1323–1330. https://doi.org/10.1016/j.ijbiomac.2020.09.232
de Paula Filho GX, Barreira TF, Pinheiro-Sant’Ana HM (2022) Chemical composition and nutritional value of three Sonchus species. Int J Food Sci 3:4181656. https://doi.org/10.1155/2022/4181656
Deng J, Xu Z, Xiang C, Liu J, Zhou L, Li T, Yang Z, Ding C (2017) Comparative evaluation of maceration and ultrasonic-assisted extraction of phenolic compounds from fresh olives. Ultrason Sonochem 37:328–334. https://doi.org/10.1016/j.ultsonch.2017.01.023
Du Z, Lu Y, Sun J, Chang K, Lu M, Fang M, Zeng X, Zhang W, Song J, Guo X, Tu P, Jiang Y (2021) Pharmacokinetics/pharmacometabolomics-pharmacodynamics reveals the synergistic mechanism of a multicomponent herbal formula, Baoyuan decoction against cardiac hypertrophy. Biomed Pharmacother 139:111665. https://doi.org/10.1016/j.biopha.2021.111665
Fu C, Yu P, Wang M, Qiu F (2020) Phytochemical analysis and geographic assessment of flavonoids, coumarins and sesquiterpenes in Artemisia annua L. based on HPLC-DAD quantification and LC-ESI-QTOF-MS/MS confirmation. Food Chem 312:126070. https://doi.org/10.1016/j.foodchem.2019.126070
Granica S, Czerwińska ME, Żyżyńska-Granica B, Kiss AK (2013) Antioxidant and anti-inflammatory flavonol glucuronides from Polygonum aviculare L. Fitoterapia 91:180–188. https://doi.org/10.1016/j.fitote.2013.08.026
Han WX, Fang JY, Reich PB, Ian Woodward F, Wang ZH (2011) Biogeography and variability of eleven mineral elements in plant leaves across gradients of climate, soil and plant functional type in China. Ecol 14:788–796. https://doi.org/10.1111/j.1461-0248.2011.01641.x
Kanaani S, Sani AM (2015) Chemical composition of essential oils and in vitro antibacterial activity of methanolic extract of Sonchus arvensis and Eremurus spectabilis against food-borne pathogenic bacteria. J Essent Oil-Bear Plants 18:1093–1099. https://doi.org/10.1080/0972060X.2014.989185
Kautsar A, Wahyuni WT, Syafitri UD, Muflihah S, Mawadah N, Rohaeti E, Arif Z, Prajogo B, Amran MB, Rohman A, Rafi M (2021) Data fusion of UV-Vis and FTIR spectra combined with principal component analysis for distinguishing of Andrographis paniculata extracts based on cultivation ages and solvent extraction. Indones J Chem 21:753–760. https://doi.org/10.22146/ijc.60321
Khan RA (2012) Evaluation of flavonoids and diverse antioxidant activities of Sonchus arvensis. Chem Cent J 6:126
Khattak KF, Rahman TR (2015) Effect of geographical distributions on the nutrient composition, phytochemical profile and antioxidant activity of Morus nigra. Pak J Pharm Sci 28:1671–1678
Khuluk RH, Yunita A, Rohaeti E, Syafitri UD, Linda R, Lim LW, Takeuchi T, Rafi M (2021) An HPLC-DAD method to quantify flavonoids in Sonchus arvensis and able to classify the plant parts and their geographical area through principal component analysis. Separations 8:12. https://doi.org/10.3390/separations8020012
Li XM, Yang PL (2018) Research progress of Sonchus species. Int J Food Prop 21:147–157. https://doi.org/10.1080/10942912.2017.1415931
Li Y, Kong D, Fu Y, Sussman MR, Wu H (2020) The effect of developmental and environmental factors on secondary metabolites in medicinal plants. Plant Physiol Biochem 148:80–89. https://doi.org/10.1016/j.plaphy.2020.01.006
Lis B, Jedrejek D, Moldoch J, Stochmal A, Olas B (2019) The anti-oxidative and hemostasis-related multifunctionality of L-chicoric acid, the main component of dandelion: an in vitro study of its cellular safety, antioxidant and anti-platelet properties, and effect on coagulation. J Funct Foods 62:103524. https://doi.org/10.1016/j.jff.2019.103524
Liu L, Zuo Z, Wang Y, Xu F (2020) A fast multi-source information fusion strategy based on FTIR spectroscopy for geographical authentication of wild Gentiana rigescens. Microchem J 159:105360. https://doi.org/10.1016/j.microc.2020.105360
Long W, Bai X, Wang S, Chen H, Yin XL, Gu HW, Yang J, Fu H (2022) UHPLC-QTOF-MS-based untargeted metabolomics and mineral element analysis insight into the geographical differences of Chrysanthemum morifolium Ramat Cv. “Hangbaiju” from different origins. Food Res Int 163:112186. https://doi.org/10.1016/j.foodres.2022.112186
Mbopi PY, Fozeng HDS, Nguekeu YMM, Bitchagno GTM, Ngantchouko CBN, Awouafack MD, Opatz T, Ngouela SA, Morita H, Tene M (2021) Chemical constituents, total phenolic content, antioxidant activity and bactericidal effect of Dicliptera verticillate (Acanthaceae). S Afr J Bot 142:216–221. https://doi.org/10.1016/j.sajb.2021.07.001
Melaku M, Tefera W (2022) Physicochemical properties, mineral and heavy metal contents of honey in Eastern Amhara Region, Ethiopia. J Food Compos Anal 114:104829. https://doi.org/10.1016/j.jfca.2022.104829
Pan H, Yao C, Yao S, Yang W, Wu W, Guo D (2020) A metabolomics strategy for authentication of plant medicines with multiple botanical origins, a case study of Uncariae rammulus Cum Uncis. J Sep Sci 43:1043–1050. https://doi.org/10.1002/jssc.201901064
Parisa N, Hidayat R, Maritska Z, Prananjaya BA (2021) Evaluation of the anti-gout effect of Sonchus arvensis on monosodium urate crystal-induced gout arthritis via anti-inflammatory action—an in vivo study. Med Pharm Rep 94:358–365. https://doi.org/10.15386/mpr-1959
Rafi M, Rismayani W, Sugiarti RM, Syafitri UD, Wahyuni WT, Rohaeti E (2021a) FTIR-based fingerprinting combined with chemometrics for discrimination of Sonchus arvensis leaf extracts and correlation with their antioxidant activity. Indones J Pharm 32:132–140. https://doi.org/10.22146/ijp.755
Rafi M, Septaningsih DA, Karomah AH, Lukman, Prajogo B, Amran MB, Rohman A (2021b) Inhibition of α-glucosidase activity, metals content, and phytochemical profiling of Andrographis paniculata from different geographical origins based on FTIR and UHPLC-Q-Orbitrap HRMS metabolomics. Biodiversitas 22:1535–1542. https://doi.org/10.13057/biodiv/d220359
Rafi M, Hasanah T, Karomah Ah, Mulyati AH, Trivadila, Rahminiwati M, Achmadi SS, Iswantini D (2022) FTIR- and UHPLC-Q-Orbitrap HRMS-based metabolomics of Sonchus arvensis extracts and evaluation of their free radical scavenging activity. Sains Malays 51:3261–3269. https://doi.org/10.17576/jsm-2022-5110-12
Raisawati T, Melati M, Aziz SA, Rafi M (2020) Total flavonoid content and antioxidant activity in leaves often cultivated Sonchus arvensis L. accessions. Plant Archives 20:1785–1792
Salazar R, Pérez-López LA, López-Arroyo J, Alanís-Garza BA, Torres NW (2011) Antimicrobial and antioxidant activities of plants from northeast of mexico. Evid Based Complement Altern Med 2011:536139. https://doi.org/10.1093/ecam/nep127
Spagnol CM, Assis RP, Brunetti IL, Isaac VLB, Salgado HRN, Corrêa MA (2019) In vitro methods to determine the antioxidant activity of caffeic acid. Spectrochim Acta Mol Biomol Spectrosc 219:358–366. https://doi.org/10.1016/j.saa.2019.04.025
Suwartiny NL, Rafi M, Rohaeti E (2022) Traditional use, phytochemical composition, and biological activities of Sonchus arvensis. Indones J Pharm 33:540–553. https://doi.org/10.22146/ijp.3823
Taslimi P, Köksal E, Gören AC, Bursal E, Aras A, Kılıç Ö, Alwasel S, Gülçin İ (2020) Anti-alzheimer, antidiabetic and antioxidant potential of Satureja cuneifolia and analysis of its phenolic contents by LC-MS/MS. Arab J Chem 13(3):4528–4537. https://doi.org/10.1016/j.arabjc.2019.10.002
Tian C, Liu X, Chang Y, Wang R, Lv T, Cui C, Liu M (2021) Investigation of the anti-inflammatory and antioxidant activities of luteolin, kaempferol, apigenin and quercetin. S Afr J Bot 137:257–264. https://doi.org/10.1016/j.sajb.2020.10.022
Wahyuni DK, Rahayu S, Zaidan AH, Ekasari W, Prasongsuk S, Purnobasuki H (2021) Growth, secondary metabolite production, and in vitro antiplasmodial activity of Sonchus arvensis L. callus under dolomite [CaMg(CO3)2] treatment. PLoS ONE 16:e0254804. https://doi.org/10.1371/journal.pone.0254804
Wang TY, Li Q, Bi K (2018) Bioactive flavonoids in medicinal plants: structure, activity and biological fate. Asian J Pharm Sci 13:12–23. https://doi.org/10.1016/j.ajps.2017.08.004
Xiao JF, Zhou B, Ressom HW (2012) Metabolite identification and quantitation in LC-MS/MS-based metabolomics. Trends Analyt Chem 32:1–14. https://doi.org/10.1016/j.trac.2011.08.009
Zhang W, Yin L, Zhao M, Tan Z, Li G (2021) Rapid and non-destructive quality verification of epoxy resin product using ATR-FTIR spectroscopy coupled with chemometric methods. Microchem J 168:106397. https://doi.org/10.1016/j.microc.2021.106397
Author Information
Department of Chemistry, Faculty of Mathematics and Natural Sciences, IPB University, Bogor, Indonesia