Pigment composition analysis of selected green microalgae through multivariate analysis and their potential as high value nutraceuticals
Research Articles | Published: 12 January, 2023
First Page: 1496
Last Page: 1508
Views: 3263
Keywords: Chemotaxonomic markers, Antioxidants activity, Pigment composition, Principal component, Factor analysis
Abstract
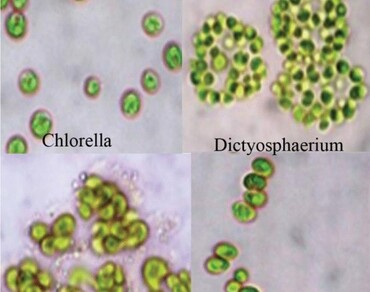
The microalgae are reported to have potential in the area of natural colours and antioxidants. In view of this, selected green microalgal strains were studied in terms of chlorophyll, carotenoids and phenolics at different days of incubation and a distinct variability was recorded for these parameters. Pigment analyses was carried out to identify the influence of these variables on each other and possible insight on providing a biomarker for communities. Mean chlorophyll content of 29.9 µg/mg dry cell wt. amongst the micro algal strains with the highest (50.4 µg/mg dry cell wt) was recorded at 14th day. Carotenoids were highest (61.3 µg/mg dry cell wt.) in Dictyosphaerium MCC 11 at 14th day, which also accounted for the highest content of phenolics (312.3 µg GAE/mg dry cell wt.). The results were grouped and correlated through multivariate analysis in order to determine which variables define and differentiate them in a better manner. Positive correlations amongst total chlorophyll and carotenoids and negative correlation between chlorophyll and phenolics were also observed. Chlorophyll and carotenoids showed greater influence on 14 days while phenolics showed greater impact at 30th day. Clustering and factor analyses of pigments outline four communities as a representation of functional types. Unique statistical relationships were found amongst studied parameters using principal component analysis and can serve as the basis of predictive models. Pigment composition and chlorophyll content depict an index of biomass and can be simplified to use taxon specific chemotaxonomic marker as a representation for biomass.
References
Afreen S, Shamsi TN, Baig MA et al (2017) A novel multicopper oxidase (laccase) from cyanobacteria: purification, characterization with potential in the decolorization of anthraquinonic dye. PLoS ONE 12:e0175144
Ampofo J, Abbey L (2022) Microalgae: bioactive composition, health benefits, safety and prospects as potential high-value ingredients for the functional food industry. Foods 11:1744. https://doi.org/10.3390/foods11121744
Andriopoulos V, Gkioni MD, Koutra E, Mastropetros SG, Lamari FN, Hatziantoniou S, Kornaros M (2022) Total phenolic content, biomass composition, and antioxidant activity of selected marine microalgal species with potential as aquaculture feed. Antioxidants 11:1320
Araujo MLV, Mendes CRB, Tavano VM, Garcia CAE, Baringer M (2017) Contrasting patterns of phytoplankton pigments and chemotaxonomic groups along 30°S in the subtropical South Atlantic Ocean. Deep-Sea Res Part I 120:112–121
Bachman RW, Horsburgh CA, Hoyer MV, Mataraza LK, Canfield DE Jr (2002) Relations between trophic state indicators and plant biomass in Florida lakes. Hydrobiologia 470:219–234
Barlow R, Gibberd M-J, Lamont T, Aiken J, Holligan P (2016) Chemotaxonomic phytoplankton patterns on the eastern boundary of the Atlantic Ocean. Deep-Sea Res I 111:73–78
Borowitzka MA (2013) High-value products from green algae—their development and commercialization. J Appl Phycol 25:743–756
Cheynier V, Comte G, Davies KM et al (2013) Plant phenolics: recent advances on their biosynthesis, genetics, and ecophysiology. Plant Physiol Biochem 72:1–20
Curcuraci E, Manuguerra S, Messina CM, Arena R, Renda G, Ioannou T, Amato V, Hellio C, Barba FJ, Santulli A (2022) Culture conditions affect antioxidant production, metabolism and related biomarkers of the microalgae Phaeodactylum tricornutum. Antioxidants 11:411
Custódio L, Justo T, Silvestre L et al (2011) Green algae of different phyla display antioxidant, metal chelating and acetylcholinesterase inhibitory activities. Food Chem. https://doi.org/10.1016/j.foodchem.2011.08.047
da Silva Ferreira V, Sant’Anna, C. (2017) Impact of culture conditions on the chlorophyll content of microalgae for biotechnological applications. World J Microbiol Biotechnol 33(1):1–8
Del Mondo A, Smerilli A, Ambrosino L, Albini A, Noonan DM, Sansone C, Brunet C (2021) Insights into phenolic compounds from microalgae: structural variety and complex beneficial activities from health to nutraceutics. Crit Rev Biotechnol 41(2):155–171
El-Agamey A, McGarvey DJ (2008) Carotenoid radicals and radical ions. In: Britton G, Liaaen-Jensen S, Pfander H (eds) Carotenoids, vol 4. Birkhäuser, Basel, pp 119–154
Ferreira V, Pinto R, Sant’Anna, C. (2016) Low light intensity and nitrogen starvation modulate the chlorophyll content of Scenedesmus dimorphus. J Appl Microbiol 1200(3):661–670
Gouveia L, Batista AP, Sousa I, Raymundo A, Bandarra NM (2008) Green algae in novel food products. In: Papadopoulos KN (ed) Food chemistry research developments: chapter 2. Nova Science Publisher Inc., New York
Grant CS, Louda JW (2010) Microalgal pigment ratios in relation to light intensity: implications for chemotaxonomy. Aquat Biol 11(2):127–138
Grubisic M (2017) A pigment composition analysis reveals community changes in preestablished stream periphyton under low-level artificial light at night. Limnologica. https://doi.org/10.1016/j.limno.2017.10.004
He Q, Yang H, Wu L, Hu C (2015) Effect of light intensity on physiological changes, carbon allocation and neutral lipid accumulation in oleaginous microalgae. Bioresour Technol 191:219–228
Hindak F (1988) Studies on the Chlorococcal algae. Biologicke Prace Bratislava 88(1–2):1–263
Hirata T, Hardman-Mountford NJ, Brewin RJW et al (2011) Synoptic relationships between surface chlorophyll a and diagnostic pigments specific to phytoplankton functional types. Biogeosciences 8:311–327
Hu J, Nagarajan D, Zhang Q, Chang JS, Lee DJ (2018) Heterotrophic cultivation of microalgae for pigment production: a review. Biotechnol Adv 36:54–67
Iyengar MOP, Desikachary TV (1981) Volvocales. Indian Council of Agricultural Research, New Delhi, p 532
Jaeschke DP, Menegol T, Rech R, Mercali GD, Marczak LDF (2016) Carotenoid and lipid extraction from Heterochlorella luteoviridis using moderate electric field and ethanol. Process Biochem 51:1636–1643
Jahnke L (1999) Massive carotenoid accumulation in Dunaliella bardawil induced by ultraviolet-A radiation. J Photoch Photobiol 48:68–74
Jaime L, Mendiola JA, Herrero M et al (2005) Separation and characterization of antioxidants from Spirulina platensis microalga combining pressurized liquid extraction, TLC, and HPLC-DAD. J Sep Sci 28:2111–2119
Klejdus B, Lojková L, Plaza M, Snóblová M, Stěrbová D (2010) Hyphenated technique for the extraction and determination of isoflavones in algae: ultrasound-assisted supercritical fluid extraction followed by fast chromatography with tandem mass spectrometry. J Chromatogr A 1217:7956–7965
Kobayashi M, Kakizono T, Nishino N, Nagai S, Kurimura Y, Tsuji Y (1997) Antioxidant role of astaxanthin in the green alga Haematococcus pluvialis. Appl Microbiol Biotechnol 48:351–356
Komárek J, Fott B (1983) The phytoplankton of the sübwassers systematics and biology. In: Elster HJ, Ohle W (eds) Part 7: chlorophyceae (green algae) order: chlorococcales. The inland waters. August Thienemann, Stuttgart, pp 1–1044
Koyande AK, Chew KW, Rambabu K, Tao Y, Chu DT, Show PL (2019) Microalgae: a potential alternative to health supplementation for humans. Food Sci Hum Wellness 80:16–24
Krienitz L, Bock C (2012) Present state of the systematics of planktonic coccoid green algae of inland waters. Hydrobiologia 698(1):295–326
Lattin JM, Carroll JD, Green PE (2003) Analyzing multivariate data. Thomson Brooks, Cole Pacific Grove
Levasseur W, Perré P, Pozzobon V (2020) A Review of high value-added molecules production by microalgae in light of the classification. Biotechnol Adv 41:1–21
Lichtenthaler HK (1987) Chlorophylls and carotenoids: pigments of photosynthetic biomembranes. Methods Enzymol 148:350–382
Manivannan K, Anantharaman P, Balasubramanian T (2012) Evaluation of antioxidant properties of marine microalga Chlorella marina (Butcher, 1952). Asian Pac J Trop Biomed 2(1):S342–S346
Martinez A, Barbosa A (2008) Antiradical power of carotenoids and vitamin E: testing the hydrogen atom transfer mechanism. J Phys Chem B 112:16945–16951
McKinney G (1941) Absorption of light by chlorophyll solutions. J Biol Chem 140:315–322
Mendes CRB, Kerr R, Tavano VM et al (2015) Cross-front phytoplankton pigments and chemotaxonomic groups in the Indian sector of the Southern Ocean. Deep-Sea Res II 118:221–232
Nagayama K, Shibata T, Fujimoto K, Honjo T, Nakamura T (2003) Algicidal effect of phlorotannin from brown alga Ecklonia kurome on red tide green algae. Aquaculture 218:601–611
Paliwal C, Ghosh T, George B, Pancha I, Maurya R, Chokshi K, Ghosh A, Mishra S (2016) Microalgal carotenoids: potential nutraceutical compounds with chemotaxonomic importance. Algal Res 15(2016):24–31
Pulz O, Gross W (2004) Valuable products from biotechnology of green algae. Appl Microbiol Biotechnol 65:635–648
Ratha SK, Prasanna R, Gupta V, Dhar DW, Saxena AK (2012) Bioprospecting and indexing the microalgal diversity of different ecological habitats of India. World J Microbiol Biotechnol 28:1657–1667
Rodrigues SV, Marinho MM, Jonk CCC et al (2014) Phytoplankton community structures in shelf and oceanic waters off southeast Brazil (20°–25°S), as determined by pigment signatures. Deep-Sea Res I 88:47–62
Serive B, Nicolau E, BeÂrard J-B, Kaas R, Pasquet V, Picot L et al (2017) Community analysis of pigment patterns from 37 microalgae strains reveals new carotenoids and porphyrins characteristic of distinct strains and taxonomic groups. PLoS ONE 12(2):e0171872. https://doi.org/10.1371/journal.pone.0171872
Silva SC, Ferreira ICFR, Dias MM, Barreiro MF (2020) Microalgae-derived pigments: a 10-year bibliometric review and industry and market trend analysis. Molecules 25(15):3406
Singleton VL, Rossi JA (1965) Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am J Enol Viticult 16:144–153
Smerilli A, Orefice I, Corato F et al (2017) Photoprotective and antioxidant responses to light spectrum and intensity variations in the coastal diatom Skeletonema marinoi. Environ Microbiol 19:611–627
Spolaore P, Joannis-Cassan C, Duran E, Isambert A (2006) Commercial applications of green algae. J Biosci Bioeng 101:87–96
Stanier RY, Kunisawa R, Mandel M, CohenBazire G (1971) Purification and properties of unicellular bluegreen algae (Order Chroococcales). Bacteriol Rev 35:171–205
Sui Y, Mazzucchi L, Acharya P, Xu Y, Morgan G, Harvey PJ (2021) A Comparison of beta-carotene, phytoene and amino acids production in Dunaliella salina DF 15 (CCAP 19/41) and Dunaliella salina (CCAP 19/30) using different light wavelengths. Foods 10:2824
Suparmi S, Fasitasari M, Martosupono M, Mangimbulude JC (2016) Comparisons of curative effects of chlorophyll from Sauropus androgynus (L) merr leaf extract and cu-chlorophyllin on sodium nitrate-induced oxidative stress in rats. J Toxicol 2016:1–7
Takaichi S (2011) Carotenoids in algae: distributions, biosyntheses and functions. Mar Drugs 9(1101):1118
Thrane J-E, Kyle M, Striebel M, Haande S, Grung M, Rohrlack T et al (2015) Spectrophotometric analysis of pigments: a critical assessment of a high-throughput method for analysis of algal pigment mixture by spectral deconvolution. Schmitt FG, editor. PLoS ONE 10(9):e0137645. https://doi.org/10.1371/journal.pone.0137645
Vishwakarma R, Dhar DW, Saxena S (2019) Influence of nutrient formulations on growth, lipid yield and biodiesel quality potential of Botryococcus sp. and Chlorella sp. Environ Sci Poll Res 26(8):7589–7600. https://doi.org/10.1007/s11356-019-04213-2
Vishwakarma R, Dhar DW, Jena M, Shukla M (2020) Biochemical parameters and 18S rRNA gene sequence analysis amongst green microalgal strains from selected aquatic sites of Eastern India. Wat Sci Technol 82(6):1205–1216. https://doi.org/10.2166/wst.2020.195
Woelfel J, Schumann R, Adler S, Hübener T, Karsten U (2007) Diatoms inhabiting a wind flat of the Baltic Sea: Species diversity and seasonal succession. Estuar Coast Shelf Sci 75:296–307
Wright SW, van den Enden RL, Pearce I, Davidson AT, Scott FJ, Westwood KJ (2010) Phytoplankton community structure and stocks in the Southern Ocean (30±80ÊE) determined by CHEMTAX analysis of HPLC pigment signatures. Deep Sea Res Part II Top Stud Oceanogr 57(9–10):758–778
Author Information
Division of Microbiology, Centre for Conservation and Utilization of Blue Green Algae, ICAR-Indian Agricultural Research Institute, New Delhi, India