RD29A-IPT expression enhances drought tolerance in transgenic perennial ryegrass
*Article not assigned to an issue yet
Research Articles | Published: 27 April, 2025
First Page: 0
Last Page: 0
Views: 648
Keywords: Accession, Isopentenyl adenosine, IPT expression, Lolium, Photochemical efficiency, RD29A promoter
Abstract
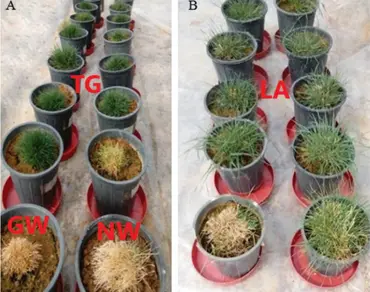
Genetic improvement and the identification of drought-tolerant cultivars are crucial in perennial ryegrass (Lolium perenne L.) turfgrass to enhance germplasm reserves for molecular breeding and the development of sustainable landscapes in arid and semi-arid green spaces. Cytokinins (CKs) are plant hormones that regulate various physiological processes, including cell division, shoot growth, and leaf senescence, and also are known to regulate plant responses to drought stress. This study aimed to enhance drought tolerance in perennial ryegrass cultivars by utilizing the drought-inducible RD29A promoter to drive the expression of the IPT gene, which boosts cytokinin levels. The research also compared the performance of these transgenic plants with wild-type (WT) plants and local perennial ryegrass accessions under varying irrigation conditions. Results showed that certain transgenic plants and local accessions displayed higher drought tolerance based on turf quality, physiological, and biochemical characteristics. The expression of the IPT gene was confirmed in transgenic plants exposed to drought stress. Transgenic lines including GM24, GM12, GC8, GC6, NC12, NC14, NS14, and GC3 exhibited increased drought tolerance, maintaining higher levels of cytokinins in the leaves, improving water content, photosynthetic rate, and antioxidant activity while reducing damage indicators. Catalase and superoxide dismutase activities were more influential than peroxidase in drought adaptation and recovery. The efficiency of the RD29A promoter and the use of the UBQ10 intron in the IPT gene construct affected gene expression. Moreover, the significant genotypic variation among local accessions indicates opportunities for improving drought tolerance through targeted breeding in sensitive and moderately tolerant genotypes. Further research is recommended to investigate hormonal balance and osmoregulation in transgenic and wild-type plants under multiple stresses. Additionally, identifying candidate genes involved in drought tolerance, particularly in local ryegrass accessions, should be a focus for future studies.
References
Alvarez S, Marsh EL, Schroeder SG, Schachtman DP (2008) Metabolomic and proteomic changes in the xylem sap of maize under drought. Plant Cell Environ 31:325–340
Arnon DI (1940) Copper enzymes in isolated chloroplasts: polyphenoloxidase in Beta vulgaris. Plant Physiol 24:1
Ayala A, Muñoz MF, Argüelles S (2014) Lipid peroxidation: production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxid Med Cell Longev 2014:360438
Bairu MW, Aremu AO, Van Staden J (2011) Somaclonal variation in plants: causes and detection methods. Plant Growth Reg 63:147–173
Barrs H, Weatherley P (1962) A re-examination of the relative turgidity technique for estimating water deficits in leaves. Aust J Biol Sci 15:413–428
Bates L, Waldren R, Teare I (1973) Rapid determination of free proline for water-stress studies. Plant Soil 39:205–207
Beznec AY (2016) Evaluación de la tolerancia a estrés hídrico en plantas de trigo (Triticum aestivum L.) con niveles de citoquininas modificados mediante transformación genética DPhil Thesis. Universidad Nacional de Luján
Beznec A, Faccio P, Miralles DJ, Abeledo LG, de Belén OCD, Garibotto M, Bossio E (2021) Stress-induced expression of IPT gene in transgenic wheat reduces grain yield penalty under drought. J Genet Eng Biotechnol 19:67
Bi A, Fan J, Hu Z, Wang G, Amombo E, Fu J, Hu T (2016) Differential acclimation of enzymatic antioxidant metabolism and photosystem II photochemistry in tall fescue under drought and heat and the combined stresses. Front Plant Sci 7:453
Blum A, Ebercon A (1981) Cell membrane stability as a measure of drought and heat tolerance in wheat 1. Crop Sci 21:43–47
Cao L, Lu X, Zhang P, Ku L, Wang G, Yuan Z, Zhang X, Cui J, Han J, Liu Y (2018) Regulatory networks of gene expression in maize (Zea mays) under drought stress and re-watering. BioRxiv 361964
Chang Z, Liu Y, Dong H, Teng K, Han L, Zhang X (2016) Effects of cytokinin and nitrogen on drought tolerance of creeping bentgrass. PLoS ONE 11:e0154005
Chaves MM, Flexas J, Pinheiro C (2009) Photosynthesis under drought and salt stress: regulation mechanisms from whole plant to cell. Ann Bot 103:551–560
DaCosta M, Huang B (2007a) Changes in antioxidant enzyme activities and lipid peroxidation for bentgrass species in response to drought stress. J Am Soc Hortic Sci 132:319–326
DaCosta M, Huang B (2007b) Drought survival and recuperative ability of bentgrass species associated with changes in abscisic acid and cytokinin production. J Am Soc Hortic Sci 132:60–66
Dhindsa RS, Matowe W (1981) Drought tolerance in two mosses: correlated with enzymatic defense against lipid peroxidation. J Exp Bot 32:79–91
Dobra J, Motyka V, Dobrev P, Malbeck J, Prasil IT, Haisel D, Gaudinova A, Havlova M, Gubis J, Vankova R (2010) Comparison of hormonal responses to heat, drought and combined stress in tobacco plants with elevated proline content. J Plant Physiol 167:1360–1370
Doumas P, Zaerr J (1988) Seasonal changes in levels of cytokinin-like compounds from Douglas-fir xylem extrudate. Tree Physiol 4:1–8
Duo LA, Liu CX, Zhao SL (2018) Alleviation of drought stress in turfgrass by the combined application of nano-compost and microbes from compost. Russ J Plant Physiol 65:419–426
Emami S, Arumainayagam D, Korf I, Rose AB (2013) The effects of a stimulating intron on the expression of heterologous genes in Arabidopsis thaliana. Plant Biotechnol J 11:555–563
Esmaeili S, Salehi H, Khosh-Khui M, Niazi A, Tohidfar M, Aram F (2019) Isopentenyl transferase (IPT) gene transfer to perennial ryegrass through sonication-assisted Agrobacterium-mediated transformation (SAAT), vacuum and heat treatment. Mol Biotechnol 61:332–344
Fariaszewska A, Staniak M (2015) Changes in yield, leaf area and fluorescence chlorophyll parameters of different forage grasses cultivars under drought stress. Acta Sci Pol Agric 14:27–38
Fu J, Huang B (2001) Involvement of antioxidants and lipid peroxidation in the adaptation of two cool-season grasses to localized drought stress. Environ Exp Bot 45:105–114
Ghandour R, Gao Y, Laskowski J, Bock R (2023) Transgene insertion into the plastid genome alters expression of adjacent native chloroplast genes at the transcriptional and translational levels. The Plant J 113:1034–1048
Hai NN, Chuong NN, Tu NHC, Kisiala A, Hoang XLT, Thao NP (2020) Role and regulation of cytokinins in plant response to drought stress. Plants 9:422
Hallmark HT, Rashotte AM (2020) Cytokinin isopentenyladenine and its glucoside isopentenyladenine-9G delay leaf senescence through activation of cytokinin-associated genes. Plant Direct 4:e00292
Hansen H, Dörffling K (2003) Root-derived trans-zeatin riboside and abscisic acid in drought-stressed and reirrigated sunflower plants: interaction in the control of leaf diffusive resistance? Funct Plant Biol 30:365–375
Havlova M, Dobrev PI, Motyka V, Štorchova H, Libus J, Dobra J, Malbeck J, Gaudinova A, Vankova R (2008) The role of cytokinins in responses to water deficit in tobacco plants over-expressing trans-zeatin O-glucosyltransferase gene under 35S or SAG12 promoters. Plant Cell Environ 31:341–353
Hiscox JD, Israelstam GF (1979) A method for the extraction of chlorophyll from leaf tissue without maceration. Can J Bot 57:1332–1334
Hu C, Delauney AJ, Verma D (1992) A bifunctional enzyme (delta 1-pyrroline-5-carboxylate synthetase) catalyzes the first two steps in proline biosynthesis in plants. Proc Natl Acad Sci 89:9354–9358
Huang B, Wang Z (2005) Physiological recovery of Kentucky bluegrass from drought stress. Intl Turfgrass Soc Res J 10:867–873
Ijaz S, Bashir A, Malik KA (2025) Expression of Agrobacterium Isopentenyl transferase (IPT) gene in wheat improves drought tolerance. Transgenic Res 34:7
Kasuga M, Miura S, Shinozaki K, Yamaguchi-Shinozaki K (2004) A combination of the Arabidopsis DREB1A gene and stress-inducible rd29A promoter improved drought-and low-temperature stress tolerance in tobacco by gene transfer. Plant Cell Physiol 45:346–350
Kishor PK, Sangam S, Amrutha R, Laxmi PS, Naidu K, Rao K, Rao S, Reddy K, Theriappan P, Sreenivasulu N (2005) Regulation of proline biosynthesis, degradation, uptake and transport in higher plants: its implications in plant growth and abiotic stress tolerance. Curr Sci 88:424–438
Koffler BE, Luschin-Ebengreuth N, Stabentheiner E, Müller M, Zechmann B (2014) Compartment-specific response of antioxidants to drought stress in Arabidopsis. Plant Sci 227:133–144
Kong X, Zhou S, Yin S, Zhao Z, Han Y, Wang W (2016) Stress-inducible expression of an F-box gene TaFBA1 from wheat enhanced the drought tolerance in transgenic tobacco plants without impacting growth and development. Front Plant Sci 7:1295
Kudoyarova GR, Vysotskaya LB, Cherkozyanova A, Dodd IC (2007) Effect of partial rootzone drying on the concentration of zeatin-type cytokinins in tomato (Solanum lycopersicum L.) xylem sap and leaves. J Exp Bot 58:161–168
Laxa M (2017) Intron-mediated enhancement: a tool for heterologous gene expression in plants? Front Plant Sci 7:1977
Liebao H, Xue L, Jun L, Huiming Z (2008) Drought-tolerant transgenic perennial ryegrass (Lolium perenne L.) obtained via particle bombardment gene transformation of CBF3/DREB1A GENE. Acta Hort 783:273–282
Man D, Bao YX, Han LB, Zhang X (2011) Drought tolerance associated with proline and hormone metabolism in two tall fescue cultivars. HortScience 46:1027–1032
Martin RC, Hollenbeck VG, Dombrowski JE (2008) Evaluation of reference genes for quantitative RT-PCR in Lolium perenne. Crop Sci 48:1881–1887
Masoabi M, Snyman S, Pols S, Hills PN, Van der Vyver C (2023) Response of sugarcane plants with modified cytokinin homeostasis under water deficit conditions. Plant Stress 10:100240
Merewitz EB, Gianfagna T, Huang B (2010) Effects of SAG12-ipt and HSP18.2-ipt expression on cytokinin production, root growth, and leaf senescence in creeping bentgrass exposed to drought stress. J Am Soc Hortic Sci 135:230–239
Merewitz EB, Gianfagna T, Huang B (2011a) Photosynthesis, water use, and root viability under water stress as affected by expression of SAG12-ipt controlling cytokinin synthesis in Agrostis stolonifera. J Exp Bot 62:383–395
Merewitz EB, Gianfagna T, Huang B (2011b) Protein accumulation in leaves and roots associated with improved drought tolerance in creeping bentgrass expressing an ipt gene for cytokinin synthesis. J Exp Bot 62:5311–5333
Merewitz EB, Belanger FC, Warnke SE, Huang B (2012a) Identification of quantitative trait loci linked to drought tolerance in a colonial× creeping bentgrass hybrid population. Crop Sci 52:1891–1901
Merewitz EB, Du H, Yu W, Liu Y, Gianfagna T, Huang B (2012b) Elevated cytokinin content in ipt transgenic creeping bentgrass promotes drought tolerance through regulating metabolite accumulation. J Exp Bot 63:1315–1328
Miao C, Zhang Y, Bai X, Qin T (2022) Insights into the response of perennial ryegrass to abiotic stress: underlying survival strategies and adaptation mechanisms. Life 12:860
Muruo RM, Nchore SB, Oduor RO, Ngugi MP (2023) Overexpressing the IPT gene improves drought tolerance and nutritional value of tropical maize (Zea mays L.). Biorxiv. https://doi.org/10.1101/2023.08.14.512900
Nguyen HN, Lai N, Kisiala AB, Emery RN (2021) Isopentenyl transferases as master regulators of crop performance: Their function, manipulation, and genetic potential for stress adaptation and yield improvement. Plant Biotechnol J 19:1297–1313
O’Brien JA, Benková E (2013) Cytokinin cross-talking during biotic and abiotic stress responses. Front Plant Sci 4:451
Oneto CD, Otegui ME, Baroli I, Beznec A, Faccio P, Bossio E, Blumwald E, Lewi D (2016) Water deficit stress tolerance in maize conferred by expression of an isopentenyltransferase (IPT) gene driven by a stress-and maturation-induced promoter. J Biotechnol 220:66–77
Patel M, Milla-Lewis S, Zhang W, Templeton K, Reynolds WC, Richardson K, Biswas M, Zuleta MC, Dewey RE, Qu R et al (2015) Overexpression of ubiquitin-like LpHUB1 gene confers drought tolerance in perennial ryegrass. Plant Biotechnol J 13:689–699
Peleg Z, Reguera M, Tumimbang E, Walia H, Blumwald E (2011) Cytokinin-mediated source/sink modifications improve drought tolerance and increase grain yield in rice under water-stress. Plant Biotechnol J 9:747–758
Pirnajmedin F, Jaškūnė K, Majidi MM (2024) Adaptive strategies to drought stress in grasses of the Poaceae family under climate change: physiological, genetic and molecular perspectives: a review. Plant Physiol Biochem. https://doi.org/10.1016/j.plaphy.2024.108814
Prerostova S, Dobrev P, Gaudinova A, Knirsch V, Körber N, Pieruschka R, Fiorani F, Brzobohaty B, Cerny M, Spichal L (2018) Cytokinins: their impact on molecular and growth responses to drought stress and recovery in Arabidopsis. Front Plant Sci 9:655
Qin H, Gu Q, Zhang J, Sun L, Kuppu S, Zhang Y, Burow M, Payton P, Blumwald E, Zhang H (2011) Regulated expression of an isopentenyltransferase gene (IPT) in peanut significantly improves drought tolerance and increases yield under field conditions. Plant and Cell Physiol 52:1904–1914
Rivero RM, Kojima M, Gepstein A, Sakakibara H, Mittler R, Gepstein S, Blumwald E (2007) Delayed leaf senescence induces extreme drought tolerance in a flowering plant. Proc Natl Acad Sci 104:19631–19636
Rivero RM, Shulaev V, Blumwald E (2009) Cytokinin-dependent photorespiration and the protection of photosynthesis during water deficit. Plant Physiol 150:1530–1540
Robson PR, Donnison IS, Wang K, Frame B, Pegg SE, Thomas A, Thomas H (2004) Leaf senescence is delayed in maize expressing the Agrobacterium IPT gene under the control of a novel maize senescence-enhanced promoter. Plant Biotechnol J 2:101–112
Su M, Wang Z, Li L, Ouyang L, Zhang Y, Liu Y (2022) Application of the fluorescent protein mCherry to study the inducible promoter rd29A under low temperature conditions in Arabidopsis. https://doi.org/10.21203/rs.3.rs-1453510/v1
Szabados L, Savoure A (2010) Proline: a multifunctional amino acid. Trends Plant Sci 15:89–97
Taleb MH, Majidi MM, Pirnajmedin F, Maibody SAMM (2023) Plant functional trait responses to cope with drought in seven cool-season grasses. Sci Rep 13:5285
Tatari M, FotouhiGhazvini R, Mousavi A, Babaei G (2018) Comparison of some physiological aspects of drought stress resistance in two ground cover genus. J Plant Nutr 41:1215–1226
Turgeon AJ (2011) Turfgrass management. Pearson Higher
Vanková R, Dobrá J, Štorchová H (2012) Recovery from drought stress in tobacco: an active process associated with the reversal of senescence in some plant parts and the sacrifice of others. Plant Signal Behav 7:19–21
Wu Y, Liu H, Wang Q, Zhang G (2021) Roles of cytokinins in root growth and abiotic stress response of Arabidopsis thaliana. Plant Growth Regul 94:151–160x
Xu Y, Tian J, Gianfagna T, Huang B (2009) Effects of SAG12-ipt expression on cytokinin production, growth and senescence of creeping bentgrass (Agrostis stolonifera L.) under heat stress. Plant Growth Regul 57:281–291
Xu L, Han L, Huang B (2011) Antioxidant enzyme activities and gene expression patterns in leaves of Kentucky bluegrass in response to drought and post-drought recovery. J Amer Soc Hort Sci 136:247–255
Xu L, Yu J, Han L, Huang B (2013) Photosynthetic enzyme activities and gene expression associated with drought tolerance and post-drought recovery in Kentucky bluegrass. Environ Exp Bot 89:28–35
Xu Y, Burgess P, Zhang X, Huang B (2016) Enhancing cytokinin synthesis by overexpressing ipt alleviated drought inhibition of root growth through activating ROS-scavenging systems in Agrostis stolonifera. J Exp Bot 67:1979–1992
Yu X, Bai G, Liu S, Luo N, Wang Y, Richmond DS, Jiang Y (2013) Association of candidate genes with drought tolerance traits in diverse perennial ryegrass accessions. J Exp Bot 64:1537–1551
Zhang X, Ervin E (2004) Cytokinin-containing seaweed and humic acid extracts associated with creeping bentgrass leaf cytokinins and drought resistance. Crop Sci 44:1737–1745
Zhang J, Kirkham M (1996) Antioxidant responses to drought in sunflower and sorghum seedlings. New Phytol 132:361–373
Zhang L, Zhao G, Xia C, Jia J, Liu X, Kong X (2012) A wheat R2R3-MYB gene, TaMYB30-B, improves drought stress tolerance in transgenic. Arabidopsis J Exp Bot 63:5873–5885
Zhang X, Ervin EH, Evanylo GK, Li J, Harich K (2013) Corn and soybean hormone and antioxidant metabolism responses to biosolids under two cropping systems. Crop Sci 53:2079–2089
Zhang X, Goatley M, Wu W, Ervin E, Shang C (2019) Drought-induced injury is associated with hormonal alteration in Kentucky bluegrass. Plant Signal Behav 14:e1651607
Author Information
Department of Horticultural Science, School of Agriculture, Shiraz University, Shiraz, Iran