Reduction of bacterial manifestation in the in vitro cultivation of Eucalyptus microcorys F. Muell
Atala Laura Ribeiro, Faria Júlio Cézar Tannure, Molinar Letícia Vaz, Avelar Maria Lopes Martins, Brondani Gilvano Ebling
Research Articles | Published: 09 March, 2022
First Page: 592
Last Page: 599
Views: 3909
Keywords: Eucalyptus , Micropropagation, Streptomycin, Sodium hypochlorite
Abstract
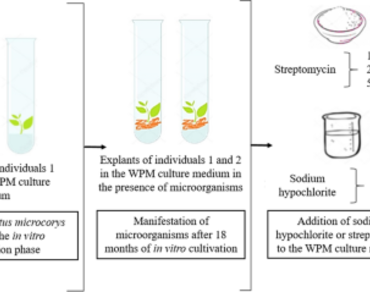
Micropropagation is one of the main applications used for tissue culture of plants. It allows rejuvenation and, consequently, improves explant rooting of woody species. However, one of its main problems is the risk of microbial manifestation. Here, we aimed to evaluate the survival and the reduction in bacterial manifestations on in vitro cultures of Eucalyptus microcorys with the supplementation of chemical agents (sodium hypochlorite or streptomycin) to WPM culture medium. We used in vitro cultivated explants from adventitious shoots of two E. microcorys selected plants, over at 44 years-old. We drew from the multiplication phase explants with 8–12 shoots that displayed bacterial manifestation and subjected them to microbe control. We added different chemical agents at different concentrations to the culture medium: sodium hypochlorite (NaOCl) and the antibiotic streptomycin. The results indicated that the best concentrations to reduce the manifestation of microorganisms during in vitro cultivation were 100 mg L−1 of streptomycin and sodium hypochlorite with 0.003% of active chlorine, since these concentrations maintained 100% survival of the explants and reduced bacterial manifestation more efficiently.
References
Bahuguna RN, Joshi R, Singh G, Shukla A, Gupta R, Bains G (2011) Micropropagation and total alkaloid extraction of Indian snake root (Rauwolfia serpentina). Indian J Agric Sci 81:1124–1129
Bavaresco LG, Pasquali R, Fluminhan A (2017) Cultivo in vitro de explantes removidos de plantas cultivadas a campo visando à micropropagação de Eucalyptus citriodora. Periód Eletr Fór “ambiental Da Alta Paulista.” https://doi.org/10.17271/1980082713620171714
Biasi LA (1995) Fitotoxicidade de três antibióticos na cultura in vitro de abacateiro. Bragantia 54:251–256. https://doi.org/10.1590/S0006-87051995000200003
Brondani GE, Dutra LF, Grossi F, Wendling I, Hornig J (2010) Estabelecimento, multiplicação e alongamento in vitro de Eucalyptus benthamii Maiden & Cambage × Eucalyptus dunnii Maiden. Rev Árvore 33:11–19. https://doi.org/10.1590/S0100-67622009000100002
Brondani GE, Araujo MAD, Alcântara BKD, Carvalho JGD, Gonçalves AN, Almeida MD (2012) In vitro organogenesis of Eucalyptus grandis: effects of boron and calcium. Acta Sci Agron 34:403–411. https://doi.org/10.4025/actasciagron.v34i4.15143
Brondani GE, de Oliveira LS, Bergonci T, Brondani AE, França FAM, da Silva ALL, Goncalves AN (2013) Chemical sterilization of culture medium: a low cost alternative to in vitro establishment of plants. Sci For 41:257–264
Cardoso JC, Imthurn ACP (2018) Esterilização química do meio de cultura para o cultivo in vitro de gérbera utilizando dióxido de cloro (ClO2). Ornam Hortic 24:218–224. https://doi.org/10.14295/oh.v24i3.1222
Chen WL, Yeh DM (2007) Elimination of in vitro contamination, shoot multiplication, and ex vitro rooting of Aglaonema. HortScience 42:629–632. https://doi.org/10.21273/HORTSCI.42.3.629
Davies FT, Geneve RL, Wilson SB, Hartamann HT, Kester DE (2017) Hartmann & Kester’s Plant propagation: principles and 280 practices, 9th edn. Pearson, London, p 1024
Dodds JH, Roberts LW (1985) Experiments in plant tissue culture. International Potato Center, Lima
Faria JCT, Terra JAP, Molinari LV, Delarmelina WM, Sena Neto AR, Carvalho D, Brondani GE (2021) Use of polylactic acid microvessel to obtain microplantlets of Eucalyptus microcorys through indirect organogenesis. 3 Biotech 11:364. https://doi.org/10.1007/s13205-021-02822-8
Ferreira EB, Cavalcanti PP, Nogueira DA (2014) ExpDes: an R package for ANOVA and experimental designs. Appl Math 5(19):2952. https://doi.org/10.4236/am.2014.519280
Furlan FC, Gavilan NH, Zorz AZ, de Oliveira LS, Konzen ER, Brondani GE (2018) Active chlorine and charcoal affect the in vitro culture of Bambusa vulgaris. Bosque 39:61–70. https://doi.org/10.4067/S0717-92002018000100061
Gavilan NH, Furlan FC, Zorz AZ, Oliveira LSD, Campos WF, Brondani GE (2018) Chemical sterilization of culture medium for in vitro multiplication of Cochlospermum regium. Cienc Rural 48:e20170581. https://doi.org/10.1590/0103-8478cr20170581
IPEF. Intituto de Pesquisas e Estudos Florestais (1984) Procedências de Eucalyptus spp. introduzidas no Brasil por diferentes entidades. Boletim Informativo IPEF volume 10 number 29. Available in: http://www.ipef.br/publicacoes/boletim_informativo/bolinf29.pdf. Accessed 15 Apr 2021
Kanjanawattanawong S, Singbumrung N, Rianthong T, Wamaedeesat R (2020) ITS2 Nucleotide sequence analysis and cleaned culture production of Butea monosperma var. lutea. Princess Naradhiwas Univ J 12:173–184
Khan T, Abbasi BH, Iqrar I, Khan MA, Shinwari ZK (2018) Molecular identification and control of endophytic contamination during in vitro plantlet development of Fagonia indica. Acta Physiol Plant 40:150. https://doi.org/10.1007/s11738-018-2727-3
Kritzinger EM, Van Vuuren RJ, Woodward B, Rong IH, Spreeth MH, Slabbert MM (1997) Elimination of external and internal contaminants in rhizomes of Zantedeschia aethiopica with commercial fungicides and antibiotics. Pathogen and microbial contamination management in micropropagation. Springer, Dordrecht, pp 161–167. https://doi.org/10.1007/978-94-015-8951-2_19
Kulkarni AP, Mahal HS, Kapoor S, Aradhya SM (2007) In vitro studies on the binding, antioxidant, and cytotoxic actions of punicalagin. J Agric Food Chem 55:1491–1500. https://doi.org/10.1021/jf0626720
Leifert C, Woodward S (1997) Laboratory contamination management; the requirement for microbiological quality assurance. Pathogen and microbial contamination management in micropropagation. Springer, Dordrecht, pp 237–244. https://doi.org/10.1007/978-94-015-8951-2_30
Leifert C, Ritchie JY, Waites WM (1991) Contaminants of plant-tissue and cell cultures. World J Microbiol Biotechnol 7:452–469. https://doi.org/10.1007/BF00303371
Lloyd G, McCown B (1980) Commercially-feasible micropropagation of mountain laurel, Kalmia latifolia, by use of shoot-tip culture. Comb Proc Int Plant Propag Soc 30:421–427
Marchesan R, de Mattos PP, Shimizu J (2005) Caracterização física, química e anatômica da madeira de Eucalyptus microcorys F. Muell. Embrapa Florestas-Comunicado Técnico (INFOTECA-E), p 133
Molinari LV, Souza DMSC, Avelar MLM, Fernandes SB, Gonçalves DS, Faria JCT, Carvalho D, Brondani GE (2021) Effects of chemical sterilization of the culture media, porous membranes and luminosity on in vitro culture of Eucalyptus grandis × Eucalyptus urophylla. J For Res 32:1587–1598. https://doi.org/10.1007/s11676-020-01240-5
Nadha HK, Salwan R, Kasana RC, Anand M, Sood A (2012) Identification and elimination of bacterial contamination during in vitro propagation of Guadua angustifolia Kunth. Pharmacogn Mag 8(30):93–97. https://doi.org/10.4103/0973-1296.96547
Nepomuceno CF, Fonseca PT, Silva TS, Oliveira LM, Santana JRF (2014) In vitro germination of Hyptis leucocephala Mart. ex Benth. and Hyptis platanifolia Mart. ex Benth. Rev Bras Pl Med 16:886–895. https://doi.org/10.1590/1983-084X/12_093
Okkels FT, Pedersen MG (1987) The toxicity to plant tissue and to Agrobacterium tumefaciens of some antibiotics. Acta Hortic 225:199–208. https://doi.org/10.17660/ActaHortic.1988.225.23
Oliveira HSD, Lemos OFD, Miranda VS, Moura HCDP, Campelo MF, Santos LRRD (2011) Estabelecimento e multiplicação in vitro de brotos no processo de micropropagação de cultivares de bananeira (Musa spp.). Acta Amazon 41:369–376. https://doi.org/10.1590/S0044-59672011000300006
Oliveira LS, Dias PC, Brondani GE (2013) Micropropagação de espécies florestais brasileiras. Pesqui Florest Bras 33(76):439–453. https://doi.org/10.4336/2013.pfb.33.76.481
Oliveira FN, Fortes GA, Paula JR, Ferri PH, Santos SC (2014) Seasonal influence on the essential oil of Eucalyptus microcorys. Nat Prod Commun 9:575–580. https://doi.org/10.1177/1934578X1400900439
Pais AK, da Silva AP, de Souza JC, Teixeira SL, Ribeiro JM, Peixoto AR, da Paz CD (2016) Sodium hypochlorite sterilization of culture medium in micropropagation of Gerbera hybrida cv. Essandre Afr J Biotechnol 15(36):1995–1998. https://doi.org/10.5897/AJB2016.15405
Paiva HN, Gomes JM (2011) Propagação vegetativa de espécies florestais, 1st edn. UFV, Viçosa, p 52
Palú EG, Corrêa LDS, Suzuki AN, Boliani AC (2011) Uso de antibióticos para o controle de bactérias endógenas visando à micropropagação da figueira. Rev Bras Frutic 33(2):587–592. https://doi.org/10.1590/S0100-29452011000200031
Pereira JES, Fortes GRDL (2003) Toxicidade de antibióticos no cultivo in vitro da batata em meios semi-sólido e líquido. Pesq Agropec Bras 38:1273–1279. https://doi.org/10.1590/S0100-204X2003001100004
Pereira GA, Ribeiro BV, Marcilio HC, Santaella MB (2011) Desinfestação e estabelecimento in vitro de explantes de bananeira ‘Grande Naine’em diferentes concentrações dehipoclorito de sódio. Rev Bras Frutic 33(spe1):222–226
Phillips R, Arnott SM, Kaplan SE (1981) Antibiotics in plant tissue culture: Rifampicin effectively controls bacterial contaminants without affecting the growth of short-term explant cultures of Helianthus tuberosus. Plant Sci Lett 21(3):235–240. https://doi.org/10.1016/0304-4211(81)90094-8
Pollock K, Barfield DG, Shields R (1983) The toxicity of antibiotics to plant cell cultures. Plant Cell Rep 2:36–39. https://doi.org/10.1007/BF00269232
Ray SS, Ali N (2017) Biotic contamination and possible ways of sterilization: a review with reference to bamboo micropropagation. Braz Arch Biol Technol. https://doi.org/10.1590/1678-4324-2016160485
Ribeiro JM, Teixeira SL, Bastos DC (2011) Cultivo in vitro de Sequoia sempervirens L. em meio de nutritivo esterilizado com hipoclorito de sódio. Ci Fl 21(1):77–82. https://doi.org/10.5902/198050982749
Ribeiro AS, de Figueiredo AJR, Tormen GCR, da Silva ALL, Campos WF, Brondani GE (2020) Clonal bamboo production based on in vitro culture. Biosci J 36(4):1261–1273. https://doi.org/10.14393/BJ-v36n4a2020-48169
Santos JD, Pinheiro MVM, Fontana DC, Schmidt D, Pretto MM (2019) Estabelecimento in vitro de oliveira ‘Arbequina’e ‘Koroneiki’. Ci Fl 29(2):508–518. https://doi.org/10.5902/1980509831305
Silva AMF, de Melo NF, de Souza EB, Coelho ADS, Marinho R (2013) Limpeza clonal de mudas de videira infectadas por Xanthomonas campestris pv. viticola. Rev Bras Frutic 35(1):316–319. https://doi.org/10.1590/S0100-29452013000100036
Souza DMSC, Fernandes SB, Silva EO, Duarte VP, Gonçalves DS, de Carvalho D, Teixeira GL, Brondani GE (2021) Effect of light intensity on in vitro introduction and multiplication of Eucalyptus grandis × Eucalyptus urophylla. In Vitro Cell Dev Biol Plant. https://doi.org/10.1007/s11627-021-10237-6 (in Press)
Teixeira SL, Teixeira MT, Ribeiro JM (2005) Chemical sterilization of culture medium: 1. culture flasks and covers-rinsing with chlorinated water. Hort Bras (suppl) 23:591
Teixeira SL, Ribeiro JM, Teixeira MT (2006) Influence of NaClO on nutrient medium sterilization and on pineapple (Ananas comosus cv Smooth cayenne) behavior. Plant Cell Tiss Organ Cult 86:375–378. https://doi.org/10.1007/s11240-006-9121-3
Teixeira SL, Ribeiro JM, Teixeira MT (2008) Utilização de hipoclorito de sódio na esterilização de meio de cultura para multiplicação in vitro de Eucalyptus pellita L. Ci Fl 18(2):185–191. https://doi.org/10.5902/19805098456
Tormen GCR, de Figueiredo AJR, Ribeiro ADS, dos Santos LF, Araujo JF, Brondani GE, da Silva ALL (2018) Carbohydrate sources, alanine and calcium for in vitro multiplication of Eucalyptus cloeziana F. Muell. Iheringia Sér Bot 73(3):329–335. https://doi.org/10.21826/2446-8231201873309
Trueman SJ, Hung CD, Wendling I (2018) Tissue culture of Corymbia and Eucalyptus. Forests 9(2):84. https://doi.org/10.3390/f9020084
Vargas DP, Formoso RS, Dutra LF, Meyer NA, dos Santos J, Ueno B (2016) Esterilização química para o cultivo in vitro de porta-enxerto de pessegueiro. Colloq Agrariae 12(1):1–6. https://doi.org/10.5747/ca.2016.v12.n1.a127
Wilson ZA, Power JB (1989) Elimination of systemic contamination in explant and protoplast cultures of rubber (Hevea brasiliensis Muell. Arg.). Plant Cell Rep 7:622–625. https://doi.org/10.1007/BF00272044
Author Information
Department of Forestry Sciences, Federal University of Lavras, Lavras, Brazil