Requirements for efficient plantlet regeneration using cotyledonary nodal explants of purple coral tree (Erythrina fusca Lour.)
Luangsriumporn Parutuch, Bodhipadma Kitti, Noichinda Sompoch, Punnakanta Luepol, Leung David W. M.
Research Articles | Published: 25 January, 2021
First Page: 37
Last Page: 41
Views: 3988
Keywords: Cotyledonary node culture, Purple coral tree, Shoot induction, Thidiazuron (TDZ)
Abstract
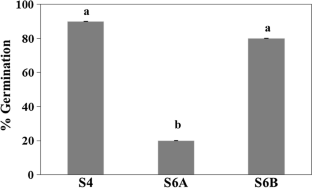
There are over hundred species in the genus Erythrina which are of practical importance in multiple ways. The requirements for Erythrina plantlet regeneration in vitro were rarely studied. Surface-sterilized seeds of purple coral tree (Erythrina fusca Lour.) exhibited a high germination rate (80%) in vitro. The cotyledonary nodes from the one-month-old purple coral tree seedlings grown in vitro were used as explants. About 70% of the cotyledonary nodal explants exhibited shoot regeneration when the explants were cultured vertically with the cut ends inserted into basal Murashige and Skoog (MS) medium compared to only 10% of those placed lying horizontally on the surface of the medium. Supplementation of the basal MS medium with 1 mg L−1 of BAP, 1 mg L−1 kinetin and 1 mg L−1 TDZ resulted in the greatest shoot elongation and the highest number of shoots developed from the cotyledonary nodal explants. The regenerated shoots were able to root (100%) on basal MS medium while supplementation with 2 mg L−1 of either NAA or IBA was inhibitory to root elongation. This is the first study on plantlet regeneration of purple coral tree which seems to have more complex requirements for shoot regeneration in vitro than those reported for the other three Erythrina species. Since only 30% of the rooted in vitro shoots survived during acclimatization following a previously published protocol for acclimatization of another plant, it is recommended that future studies are to investigate the requirements of higher plantlet survival for the rooted in vitro shoots of purple coral tree.
References
- Benson EE (2000) Special symposium: in vitro plant recalcitrance: an introduction. Vitro Cell Dev Biol Plant 36:141–148
- Bodhipadma K, Noichinda S, Luangsriumporn P, Meenapa C, Nathalang K, Leung DWM (2012) Ginger juice enhanced growth of aromatic chilli during in vitro culture and acclimatization. J Appl Horticult 14:88–91
- Chand S, Singh AK (2004) In vitro shoot regeneration from cotyledonary node explants of a multipurpose leguminous tree, Pterocarpus marsupium Roxb. Vitro Cell Dev Biol Plant 40:167–170
- da Costa GM, Nepomuceno CF, de Santana JRF (2010) In vitro propagation of Erythrina velutina. Cienc Rural 40:1090–1096
- de Araújo-Júnior JX, de Oliveira MSG, Aquino PGV, Alexandre-Moreira MS, Sant’Ana AEG (2012) A phytochemical and ethnopharmacological: review of the genus Erythrina. In: Rao V (ed) Phytochemicals–a global perspective of their role in nutrition and health. InTech, Croatia, pp 327–352
- Fonseca PT, Nepomuceno CF, Alvim BFM, Santana JRF (2014) Respostamorfogênica de embriõeszigóticos de Erythrina velutina Willd: (Leguminosae) cultivados in vitro. Rev Ceres 61:605–611
- Garcı´a-Mateos R, Soto-Herna´ndez M, Martı´nez-Va´zquez M, Villegas-Monter A (1999) Isolation of alkaloid of Erythrina from tissue culture. Phytochem Anal 10:12–16
- García-Mateos MR, Soto-Hernández RM, Gutiérrez RJM, Villegas-Monter A (2005) Alkaloids from several subcultures of Erythrina americana Miller. calluses. Rev Chapingo Ser Hortic 11:21–26
- Ghosh PK, Maiti TK (2014) Effect of seed scarification on in vitro seed germination of Abrus precatorius L. Plant Arch 14:881–885
- Islam S, Wetten A (1999) In vitro regeneration of Erythrina variegata, a multipurpose fast-growing tree in Bangladesh. Plant Tissue Cult 9:97–105
- Javed SB, Anis M (2015) Cobalt induced augmentation of in vitro morphogenic potential in Erythrina variegata L.: a multipurpose tree legume. Plant Cell Tissue Org Cult 120:463–474
- Javed SB, Alatar AA, Anis M, Faisal M (2017) Synthetic seeds production and germination studies, for short term storage and long distance transport of Erythrina variegata L.: a multipurpose tree legume. Ind Crop Prod 105:41–46
- Khaonim K, Zongram O, Palanuvej C, Ruangrungsi N (2017) Microscopic characterization of Erythrina species distributed in Thailand. J Sci Tech UBU Suppl Ed 52–59
- Kirika MW, Kahia JW, Diby LN, Njagi EM, Dadjo C, Kouame C (2015) Micropropagation of an endangered medicinal and indigenous multipurpose tree species: Erythrina abyssinica. HortSc 50:738–743
- Kumar N, Reddy MP (2010) Plant regeneration through the direct induction of shoot buds from petiole explants of Jatropha curcas: a biofuel plant. Ann Appl Biol 156(3):367–375
- Maity A, Chakraborti D, Chaudhuri RK (2014) An efficient in vitro regeneration strategy in a recalcitrant grain legume Vigna radiata (L.) Wilczek. Heritage 1:129–132
- Maldonado-Arciniegas F, Ruales C, Caviedes M, Ramírez DX, León-Reyes A (2018) An evaluation of physical and mechanical scarification methods on seed germination of Vachellia macracantha (Humb. & Bonpl. ex Willd.) Seigler & Ebinger. Acta Agron 67:120–125
- Marín NB (2002) Erythrina fusca Lour. (Part II—species descriptions). In: Vozzo JA (ed) Tropical tree seed manual. USDA, Washington DC, pp 458–460
- Molina SM, Trujillo MI (1999) Techniques for in vitro seed germination in Pistacia species. S Afr J Bot 65:149–152
- Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:473–497
- Neill DA (1993) Botany and ecology. In: Powell MH, Westley SB (eds) Erythrina production and use: a field manual. Nitrogen Fixing Tree Association, Paia, Hawaii, pp 2–6
- Orwa C, Mutua A, Kindt R, Jamnadass R, Simons A (2009) Erythrina fusca. Agroforestree database: a tree reference and selection guide version 4.0, pp 1–5
- Prando MAS, Chiavazza P, Faggio A, Contessa C (2014) Effect of coconut water and growth regulator supplements on in vitro propagation of Corylus avellana L. Sci Hortic 171:91–94
- San Miguel-Ch´avez R, Soto-Hern´andez M, Ramos-Valdivia AC, Kite G (2007) Alkaloid production in elicited cell suspension cultures of Erythrina americana Miller. Phytochem Rev 6:167–173
- Shasthree T, Imran MA, Mallaiah B (2009) In vitro rooting from callus cultures derived from seedling explants of Erythrina variegata L. Curr Trends Biotechnol Pharm 3:447–452
- Shyamkumar B, Anjaneyulu C, Giri C (2003) Multiple shoot induction from cotyledonary node explants of Terminalia chebula. Biol Plant 47:585–588
- Stevens ME, Pijut PM (2018) Rapid in vitro shoot multiplication of the recalcitrant species Juglans nigra L. Vitro Cell Dev Biol Plant 54:309–317
Author Information
Division of Agro-Industrial Technology, Faculty of Applied Science, King Mongkut’s University of Technology North Bangkok, Bangkok, Thailand