Silver nanoparticle-induced shikonin production in cell cultures of Arnebia benthamii (Wall ex. G. Don) Johnston
*Article not assigned to an issue yet
Joshi Namini, Rawat Balwant, Sinha Somya, Negi Shivani, Raj Akshita, Agarwal Mrinalini, Rai Nishant, Rawat Janhvi Mishra, Rawat Balwant, Sinha Somya
Research Articles | Published: 24 June, 2025
First Page: 0
Last Page: 0
Views: 750
Keywords: n Arnebia benthamiin , Nanoparticles, Antioxidant activity, Elicitors, Shikonin, Callus
Abstract
Secondary metabolites hold significant commercial value due to their diverse applications in the pharmaceutical industry. Controlled elicitation is a widely used strategy to enhance the biosynthesis of these compounds in plant cultures. The present study aimed to evaluate the effect of silver nanoparticles (AgNPs) as a novel elicitor to enhance the production of bioactive secondary metabolites, including shikonin, in the cell suspension culture of Arnebia benthamii. Callus was induced on Murashige and Skoog (MS) medium supplemented with 2.5 µM Thidiazuron (TDZ) and 0.5 µM 1-Napthalene acetic acid (NAA) with 86.43 ± 4.22 percent callus induction frequency. Further, suspension culture was established by transferring 0.50 g callus to MS liquid medium having the same PGRs combination (2.5 µM TDZ and 0.5 µM NAA) and exposed to varying concentrations of AgNPs (20–80 µg/L). The impact on biomass accumulation, total phenolic content, flavonoid content, tannin content, and shikonin production was assessed. Antioxidant potential was analyzed using DPPH (2,2-diphenyl-1-picrylhydrazyl), ABTS (2,2′-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid)) and FRAP (ferric ion reducing antioxidant potential) in vitro assays. The addition of 40 µg/L AgNPs resulted in the highest callus biomass (3.89 ± 0.45 g fresh weight) and significantly enhanced the accumulation of phenolics, flavonoids, and tannins. However, maximum shikonin production was observed at 60 µg/L AgNPs. The antioxidant activity was also highest at 40 µg/L AgNPs, with a DPPH scavenging potential of 89.56 ± 2.06%. It may be concluded that silver nanoparticles significantly influence biomass proliferation and secondary metabolite biosynthesis in A. benthamii suspension cultures. The optimized elicitation protocol offers a promising strategy for sustainable and scalable production of bioactive metabolites, potentially reducing the harvesting pressure on natural populations of this endangered medicinal plant.
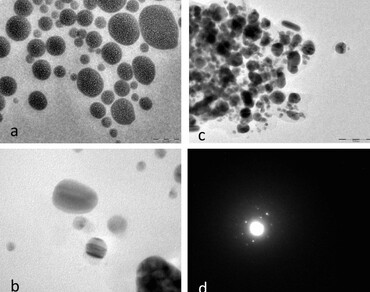
References
Abbasi BH, Khan M, Guo B (2011) Efficient regeneration and antioxidative enzyme activities in Brassica rapa var. turnip. Plant Cell Tiss Organ Cult 105:337–344
Abdelkawy AM, Alshammari SO, Hussein HAA, Abou El-Enain IM, Abdelkhalek ES, Radwan AM, Kenawy SKM, Maaty DAM, Abed NN, Sabry S, Mohsen A (2023) Effect of silver nanoparticles on tropane alkaloid production of transgenic hairy root cultures of Hyoscyamus muticus L. and their antimicrobial activity. Sci Rep 13(1):10397
AbuDalo MA, Al-Mheidat IR, Al-Shurafat AW, Grinham C, Oyanedel-Craver V (2019) Synthesis of silver nanoparticles using a modified Tollens’ method in conjunction with phytochemicals and assessment of their antimicrobial activity. PeerJ 7:e6413
Ahmad MA, Javed R, Adeel M, Rizwan M, Ao Q, Yang Y (2020) Engineered ZnO and CuO nanoparticles ameliorate morphological and biochemical response in tissue culture regenerants of candyleaf (Stevia rebaudiana). Molecules 25(6):1356. https://doi.org/10.3390/molecules25061356
Ali A, Mashwani ZUR, Raja NI, Mohammad S, Luna-Arias JP, Ahmad A, Kaushik P (2023) Phytomediated selenium nanoparticles and light regimes elicited in vitro callus cultures for biomass accumulation and secondary metabolite production in Caralluma tuberculata. Front Plant Sci 14:1253193
Ali A, Mohammad S, Khan MA, Raja NI, Arif M, Kamil A, Mashwani ZR (2019) Silver nanoparticles elicited in vitro callus cultures for accumulation of biomass and secondary metabolites in Caralluma tuberculate. Artif Cells Nanomed Biotechnol 47(1):715–724. https://doi.org/10.1080/21691401.2019.1577884
Anjum S, Komal A, Abbasi BH, Hano C (2021) Nanoparticles as elicitors of biologically active ingredients in plants. Nanotechnol Plant Growth Promot Prot Recent Adv Impacts 6:170–202
Ayoola-Oresanya IO, Sonibare MA, Gueye B, Abberton MT, Morlock GE (2021) Elicitation of antioxidant metabolites in Musa species in vitro shoot culture using sucrose, temperature and jasmonic acid. Plant Cell Tiss Organ Cult 146:225–236. https://doi.org/10.1007/s11240-021-02062-x
Brand-Williams W, Cuvelier ME, Berset CLWT (1995) Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci Technol 28(1):25–30
Bosila H, Hamza MA, El-Ateeq AAEF (2016) Enhancement of callus growth and hyoscyamine alkaloid production in Hyoscyamus muticus by nanotechnology, biotic elicitor and precursor. Int J Chem Tech Res 9(7):135–142
Cai Y, Luo Q, Sun M, Corke H (2004) Antioxidant activity and phenolic compounds of 112 traditional Chinese medicinal plants associated with anticancer. Life Sci 74(17):2157–2184
Chandran H, Meena M, Barupal T, Sharma K (2020) Plant tissue culture as a perpetual source for production of industrially important bioactive compounds. Biotechnology Reports 26:e00450
Coste A, Vlase L, Halmagyi A, Deliu C, Coldea G (2011) Effects of plant growth regulators and elicitors on production of secondary metabolites in shoot cultures of Hypericum hirsutum and Hypericum maculatum. Plant Cell, Tissue Organ Cult 106:279–288
Danu K (2016). In vitro micropropagation of Paris polyphylla Smith: an endangered medicinal plant of Himalayan region. Ph.D. Thesis, Kumaun University, Nainital, Uttarakhand, India
Ebadollahi R, Jafarirad S, Kosari-Nasab M, Mahjouri S (2019) Effect of explant source, perlite nanoparticles and TiO2/perlite nanocomposites on phytochemical composition of metabolites in callus cultures of Hypericum perforatum. Sci Rep 9(1):12998
Fazal H, Abbasi BH, Ahmad N, Ali M, Shujait Ali S, Khan A, Wei DQ (2019) Sustainable production of biomass and industrially important secondary metabolites in cell cultures of selfheal (Prunella vulgaris L.) elicited by silver and gold nanoparticles. Artif Cells Nanomed Biotechnol 47(1):2553–2561. https://doi.org/10.1080/21691401.2019.1625913
Golkar P, Bakhtiari MA, Bazarganipour M (2021) The effects of nanographene oxide on the morpho-biochemical traits and antioxidant activity of Lepidium sativum L. under in vitro salinity stress. Sci Hortic 288:110301. https://doi.org/10.1016/j.scienta.2021.110301
Guo C, He J, Song X, Tan L, Wang M, Jiang P, Li Y, Cao Z, Peng C (2019) Pharmacological properties and derivatives of shikonin—A review in recent years. Pharmacol Res 149:104463
Gupta K, Garg S, Singh J, Kumar M (2014) Enhanced production of napthoquinone metabolite (shikonin) from cell suspension culture of Arnebia sp. and it’s up-scaling through bioreactor. 3 Biotech 4:263–273
Hong PI, Chen JH, Chang WC (2008) Plant regeneration via protocorm like body formation and shoot multiplication from seed derived callus of a maudia type slipper orchid. Acta Physiol Plant 30:755–759
Jalalpour Z, Shabani L, Afghani KL, Sharifi-Tehrani M, Amini SA (2014) Stimulatory effect of methyl jasmonate and squalestatin on phenolic metabolism through induction of LOX activity in cell suspension culture of yew. Turk J Biol. https://doi.org/10.3906/biy-1306-91
Jasim B, Thomas R, Mathew J, Radhakrishnan EK (2017) Plant growth and diosgenin enhancement effect of silver nanoparticles in Fenugreek (Trigonella foenumgraecum). Saudi Pharm J 25(3):443–447. https://doi.org/10.1016/j.jsps.2016.09.012
Kalisz A, Húska D, Jurkow R, Dvořák M, Klejdus B, Caruso G, Sękara A (2021) Nanoparticles of cerium, iron, and silicon oxides change the metabolism of phenols and flavonoids in butterhead lettuce and sweet pepper seedlings. Environ Sci Nano 8(7):1945–1959
Kasote DM, Katyare SS, Hegde MV, Bae H (2015) Significance of antioxidant potential of plants and its relevance to therapeutic applications. Int J Biol Sci 11(8):982
Kavianifar S, Ghodrati K, Naghdi Badi H, Etminan AR (2018) Effects of nano elicitors on callus induction and mucilage production in tissue culture of Linum usitatissimum L. J Med Plants 17(67):45–54
Khodakovskaya MV, De Silva K, Biris AS (2012) Carbon nanotubes induce growth enhancement of tobacco cells. ACS Nano 6:2128–2135
Kocot D, Nowak B, Sitek E, Starzyńska-Janiszewska A, Mitka J (2022) In vitro shoot regeneration from organogenic callus culture and rooting of Carpathian endemic Aconitum bucovinense Zapał. Plant Cell Tissue Organ Cult (PCTOC) 151(1):177–187
Kruszka D, Selvakesavan RK, Kachlicki P, Franklin G (2022) Untargeted metabolomics analysis reveals the elicitation of important secondary metabolites upon treatment with various metal and metal oxide nanoparticles in Hypericum perforatum L. cell suspension cultures. Ind Crop Prod 178:114561
Kumar S, Dobos GJ, Rampp T (2017) The significance of ayurvedic medicinal plants. J Evid Based Complementary Altern Med 22(3):494–501. https://doi.org/10.1177/2156587216671392
Kumar A, Nirmal P, Kumar M, Jose A, Tomer V, Oz E, Proestos C, Zeng M, Elobeid T, Sneha K, Faith Oz (2023) Major phytochemicals: recent advances in health benefits and extraction method. Molecules 28(2):887. https://doi.org/10.3390/molecules28020887
Lala S (2020) Enhancement of secondary metabolites in Bacopa monnieri (L.) Pennell plants treated with copper-based nanoparticles. IET Nanobiotechnol 14(1):78–85
Lepcha DL, Chhetri A, Chhetri DR (2019) Antioxidant and cytotoxic attributes of Paris polyphylla Smith from Sikkim Himalaya. Pharmacogn J 11(4):705–711
Malik S, Bhushan S, Sharma M, Ahuja PS (2011) Physico-chemical factors influencing the shikonin derivatives production in cell suspension cultures of Arnebia euchroma (Royle) Johnston, a medicinally important plant species. Cell Biol Int 35(2):153–158
Marslin G, Sheeba CJ, Franklin G (2017) Nanoparticles alter secondary metabolism in plants via ROS burst. Front Plant Sci 8:832. https://doi.org/10.3389/fpls.2017.00832
Mishra N, Jiang C, Chen L, Paul A, Chatterjee A, Shen G (2023) Achieving abiotic stress tolerance in plants through antioxidative defense mechanisms. Front Plant Sci 14:1110622
Moharrami F, Hosseini B, Sharafi A, Farjaminezhad M (2017) Enhanced production of hyoscyamine and scopolamine from genetically transformed root culture of Hyoscyamus reticulatus L. elicited by iron oxide nanoparticles. In Vitro Cell Dev Biol Plant 53:104–111
Moola AK, Senthil Kumar T, Ranjitha Kumari BD (2022) Enhancement of Celastrol compound by silver nanoparticles and acetosyringone in Celastrus paniculatus Willd. through adventitious and hairy root culture. J Plant Biochem Biotechnol 31(2):429–434
Mosavat N, Golkar P, Yousefifard M, Javed R (2019) Modulation of callus growth and secondary metabolites in different Thymus species and Zataria multiflora micropropagated under ZnO nanoparticles stress. Biotechnol Appl Biochem 66(3):316–322. https://doi.org/10.1002/bab.1727
Mosavat N, Yousefifard M, Golkar P, Javed R (2022) Influence of Ag nanoparticles on physiological and biochemical aspects of callus of Thymus species and Zataria multiflora Boiss. Acta Agricu Slovenica 118(3):1–8
Murashige T, Skoog F (1962) A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plant 15(3):473–497
Namdeo AG (2007) Plant cell elicitation for production of secondary metabolites: a review. Pharmacogn Rev 1(1):69–79
Oyaizu M (1986) Studies on products of browning reaction: antioxidant activity of products of browning reaction prepared from glucosamine. Jap J Nutr 44:307–315
Pant M, Chankeshwara NN, Rathod A, Husen A (2024) Tissue culture techniques for medicinal plant propagation. Tissue culture techniques and medicinal plants. CRC Press, Boca Raton, pp 1–16
Parray J, Kamili A, Jan S, Mir M, Nowsheen S, Bashir B, Allah A, Fathi E, Abeer H, Abdulaziz A (2018) Manipulation of plant growth regulators on phytochemical constituents and DNA protection potential of the medicinal plant Arnebia benthamii (Wall. ex G. Don) Johnston. Biomed Res Int. https://doi.org/10.1155/2018/6870139
Purohit S, Rawat V, Jugran AK, Singh RV, Bhatt ID, Nandi SK (2015) Micropropagation and genetic fidelity analysis in Valeriana jatamansi Jones. J Appl Res Med Aromat Plants 2(1):15–20
Purohit S, Rawat JM, Pathak VK, Singh DK, Rawat B (2021) A hydroponic-based efficient hardening protocol for in vitro raised commercial kiwifruit (Actinidia deliciosa). In Vitro Cell Dev Biol-Plant 57(3):541–550
Quadri RR, Kamili AN, Silva JT, Shah AM (2012) In vitro multiplication of Arnebia benthamii Wall., a critically endangered medicinal herb of the Western Himalayas. Med Aromat Plant Sci Biotechnol 6(1):54–57
Ramirez-Estrada K, Vidal-Limon H, Hidalgo D, Moyano E, Golenioswki M, Cusidó RM, Palazon J (2016) Elicitation, an effective strategy for the biotechnological production of bioactive high-added value compounds in plant cell factories. Molecules 21(2):182
Rawat JM, Rawat B, Agnihotri RK, Chandra A, Nautiyal S (2013) In vitro propagation, genetic and secondary metabolite analysis of Aconitum violaceum Jacq.: a threatened medicinal herb. Acta Physiol Plant 35:2589–2599
Rawat JM, Bhandari A, Mishra M, Rawat B, Kumar A, Thakur A, Chandra A (2018) Genetic stability and phytochemical profiling of the in vitro regenerated plants of Angelica glauca Edgew.: an endangered medicinal plant of Himalaya. Plant Cell Tiss Organ Cult. https://doi.org/10.1007/s11240-018-1448-z
Rawat V, Ghildiyal A, Singh L, Jugran AK, Bhatt ID, Nandi SK, Pande V (2019) Methyl jasmonate induced polyphenols and antioxidant production in callus suspension culture of Nardostachys jatamansi. Plant Biosyst Int J Deal Aspects Plant Biol. https://doi.org/10.1080/11263504.2019.1701124
Rawat JM, Pandey S, Rawat B, Purohit S, Anand J, Negi AS, Thakur A, Mahmoud MH, El-Gazzar AM, El-Saber Batiha G (2023a) In vitro production of steroidal saponin, total phenols and antioxidant activity in callus suspension culture of Paris polyphylla Smith: an important Himalayan medicinal plant. Front Plant Sci 14:1225612. https://doi.org/10.3389/fpls.2023.1225612
Rivero-Montejo SDJ, Vargas-Hernandez M, Torres-Pacheco I (2021) Nanoparticles as novel elicitors to improve bioactive compounds in plants. Agriculture 11(2):134
Shakya S, He Y, Ren X, Guo T, Maharjan A, Luo T, Wang T, Dhakhwa R, Regmi B, Li H, Gref R, Zhang J (2019) Ultrafine silver nanoparticles embedded in cyclodextrin metal-organic frameworks with GRGDS functionalization to promote antibacterial and wound healing application. Small 15(27):1901065
Sharma P, Bhatt D, Zaidi MGH, Saradhi PP, Khanna PK, Arora S (2012) Silver nanoparticle-mediated enhancement in growth and antioxidant status of Brassica juncea. Appl Biochem Biotechnol 167(8):2225–2233. https://doi.org/10.1007/s12010-012-9759-8
Srinivasan K (2007) Black pepper and its pungent principle-piperine: a review of diverse physiological effects. Crit Rev Food Sci Nutr 47:735–748
Surveswaran S, Cai YZ, Corke H, Sun M (2007) Systematic evaluation of natural phenolic antioxidants from 133 Indian medicinal plants. Food Chem 102(3):938–953
Thakur M, Bhattacharya S, Khosla PK, Puri S (2019) Improving production of plant secondary metabolites through biotic and abiotic elicitation. J Appl Res Med Aromat Plants 12:1–12
Yadav S, Sharma A, Nayik GA, Cooper R, Bhardwaj G, Sohal HS, Mutreja V, Kaur R, Areche FO, AlOudat M, Shaikh AM, Kovacs B, Ahmed AEM (2022) Review of shikonin and derivatives: isolation, chemistry, biosynthesis, pharmacology and toxicology. Front Pharmacol 13:905755
Zahir A, Nadeem M, Ahmad W et al (2019) Chemogenic silver nanoparticles enhance lignans and neolignans in cell suspension cultures of Linum usitatissimum L. Plant Cell Tiss Organ Cult 136:589–596. https://doi.org/10.1007/s11240-018-01539-6
Zaka M, Abbasi BH, Rahman L-U, Shah A, Zia M (2016) Synthesis and characterisation of metal nanoparticles and their effects on seed germination and seedling growth in commercially important Eruca sativa. IET Nanobiotechnol 10(3):1–7. https://doi.org/10.1049/ietnbt.2015.0039
Zhang B, Zheng LP, Yi Li W, Wen Wang J (2013) Stimulation of artemisinin production in Artemisia annua hairy roots by Ag-SiO2 core-shell nanoparticles. Curr Nanosci 9(3):363–370
Author Information
Department of Biotechnology, Graphic Era Deemed to Be University, Dehradun, India