Alleviation of nickel toxicity by molybdenum in wheat
Research Articles | Published: 05 August, 2024
First Page: 1970
Last Page: 1980
Views: 2258
Keywords: Nickel effects, Nickel–molybdenum interaction, Antioxidants, Wheat seedling, Nitric oxide
Abstract
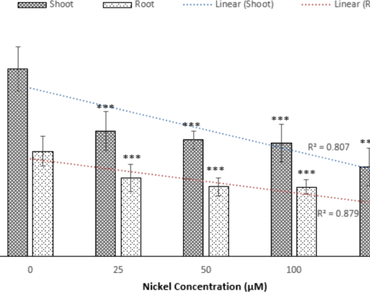
Heavy metal contamination in soil adversely affects wheat productivity. Trace amounts of nickel (Ni) and molybdenum (Mo) are required in the soil for plant metabolism and growth. At high concentrations, Ni is strongly toxic to acidic plants. Present study investigated the effects of Ni (25–200 µM) and combination of Ni (100 µM) with Mo (0.5; T4 and 2 µM; T5) on antioxidative parameters under acidic conditions (pH 5.0) using Triticum durum var. HI8737 seedlings. A supply of 25–200 µM NiCl3 resulted in a significant reduction of fresh tissue weight, which was more substantial for the former. Furthermore, Ni treatment led to enhanced oxidative stress, as reflected by increased MDA and H2O2 levels in the tissue. In addition to CAT, antioxidant enzymes respond well to stress, up to 100 µM Ni in roots. SOD and APX increased significantly from 25 to 100 µM, while a significant increase in Gu-POX activity was observed only at 100 µM. For the shoots, a significant decrease was observed, while that of APX was not significantly different. The application of Mo with Ni resulted in a significant increase of plant growth in terms of fresh weight. Significant reductions in lipid peroxidation and H2O2 content were also observed in T4 and T5. The interactive effect of Ni-Mo had intermediate effects on SOD and APX activities in T4 treatment and on CAT activity in T5 treatment. A significant increase in NO content and increased NR activity in the roots suggests the role of Mo in overcoming Ni stress.
References
Aebi H (1984) Catalase in vitro. In: Packer L (ed) Methods in enzymology, vol 105. Academic, Orlando, pp 121–126
Beauchamp C, Fridovich I (1971) Superoxide dismutase: improved assay and an assay applicable to acrylamide gels. Annals Biochem 44:276–287
Boer JL, Mulrooney SB, Hausinger RP (2014) Nickel-dependent metalloenzymes. Arch of Biochem Biophy 544:142–152
Corpas FJ, Río LA, Palma JM (2019) Impact of nitric oxide (NO) on the ROS metabolism of peroxisomes. Plants 8(2):37
Gajewska E, Skłodowska M (2007) Effect of nickel on ROS content and antioxidative enzyme activities in wheat leaves. Bio Metals 20:27–36
Gajewska E, Skłodowska M (2008) Differential biochemical responses of wheat shoots and roots to nickel stress: antioxidative reactions and proline. Plant Growth Regul 54:179–188
Gajewska E, Skłodowska M (2010) Diffential effect of equal copper, cadmium and nickel concentration on biochemical reaction in wheat seedlings. Exotoxic Environ 73:993–1003
Gajewska E, Słaba M, Andrzejewska R et al (2006a) Nickel-induced inhibition of wheat root growth is related to h2o2 production, but not to lipid peroxidation. Plant Growth Regul 49:95–103
Gajewska E, Skłodowska M, Słaba M, Mazur J (2006b) Effect of nickel on antioxidative enzyme activities, proline and chlorophyll contents in wheat shoots. Biologia Planta 50(4):653–659
Gajewska E, Wielanek M, Bergier K, Skłodowska M (2009) Nickel-induced depression of nitrogen assimilation in wheat roots. Acta Physiol Planta 31:1291–1300
Gajewska E, Drobik D, Wielanek M, Nalewajko SJ, Gocawski J, Mazur Z, Skłodowska M (2013) Alleviation of nickel toxicity in wheat (Triticum aestivum L.) seedlings by selenium supplementation. Biol Lett 50(2):63–76
Gajewska E, Bernat P, Długonski J, Skłodowska M (2012) Effect of nickel on membrane integrity, lipid peroxidation and fatty acid composition in wheat seedlings. J Agron Crop Sci 1–9
Gimeno-Garcia E, Andreu V, Boluda R (1996) Heavy metals incidence in the application of inorganic fertilizers and pesticides to rice farming soils. Environ Pol 92:19–25
Gonnelli C, Galardi F, Gabbrielli R (2001) Nickel and copper tolerance in three tuscan populations of Silene paradoxa. Physiol Planta 113:507–514
Hodges DM, Delong JM, Forney CF, Prange RK (1999) Improving the thiobarbituric acid-reactive-substances assay for estimating lipid peroxide in plant tissues containing anthocyanin and other interfering compounds. Planta 207:604–611
Lowry OH, Rosebrough NJ, Farr LJ, Randall RJ (1951) Protein measurement with the folin phenol reagent. J Biol Chem 193:265–275
Lübben S, Sauerbeck D (1991) The uptake and distribution of heavy metals by spring wheat. Water Air Soil Pol 57–58(1):239–247
Magdalena A, Jolanta FW (2007) Nitric oxide as a bioactive signaling molecule in plant stress responses. Plant Sci 172:876–887
Marschner H (1995) Mineral nutrition of higher plants, 2nd edn. Academic Press, Toronto
Matraszek R, Hawrylak-Nowak B, Chwil S et al (2016) Macronutrient composition of nickel-treated wheat under different sulfur concentrations in the nutrient solution. Environ Sci Pollut Res 23:5902–5914
Nakano Y, Asada K (1981) Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol 22(5):867–880
Nie Z, Li S, Hu C, Sun X, Tan Q, Liu H (2015) Effects of molybdenum and phosphorus fertilizers on cold resistance in winter wheat. J Plant Nut 38:808–820
Pandolfini T, Gabbrielli R, Comparini C (1992) Nickel toxicity and peroxidize activity in seedlings of Triticum aestivum L. Plant Cell Environ 15:719–725
Pedregosa CM, Reyes GJA, Canadillas M, Navas P, Cordoba F (1996) Role of apoplastic and cell-wall peroxidases on the stimulation. Plant Physiol 112:1119–1125
Putter J (1974) Peroxidases. In: Bergmeyer HU (ed) Methods of enzymatic analysis, vol. 2. Weinheim/New York: Verlag Chemie/Academic Press, 685–690
Rio LA, Corpas FJ, Sandalio LM, Palma JM, Barroso JB (2003) Plant peroxisomes, reactive oxygen metabolism and nitric oxide. UBMB Life 55:71–81
Sönmez D, Doğan M (2020) Effects of chromium, nickel and their combined applications on Triticum aestivum L. in germination stage. Acta Biol Turcica 33(1):26–34
Srivastava HS (1975) Distribution of nitrate reductase in bean seedlings. Plant Cell Physiol 16:995–999
Sun X, Hu C, Tan Q (2006) Effects of molybdenum on antioxidative defense system and membrane lipid peroxidation in winter wheat under low temperature stress. J Plant Physiol Mol Biol 32:175–182
Szwarc W, Skórska E, Węgrzecki A (2006) Comparison of the response of wheat and oats to nickel in subsoil during the early phase of growth. Polish J Environ Stud 15:195–198
Velikova V, Yordanov I, Edreva RA (2000) Oxidative stress and some antioxidants in acid rain- treated bean plants. Plant Sci 151:59–66
Wu S, Hu C, Tan Q, Nie Z, Sun X (2014) Effects of molybdenum on water utilization, antioxidative defense system and osmotic-adjustment ability in winter wheat (Triticum aestivum) under drought stress. Plant Physiol Biochem 83:365–374. https://doi.org/10.1016/j.plaphy.2014.08.022
Wu S, Hu C, Tan Q, Xu S, Sun W (2017) Nitric oxide mediates molybdenum-induced antioxidant defense in wheat under drought stress. Front Plant Sci 8:1–11
Yamasaki H, Sakihama Y (1999) Simultaneous production of nitric oxide and peroxynitrite by plant nitrate reductase: In vivo evidence for the NR-dependent formation of active nitrogen species. FEBS Lett 468:89–92
Yaneva I, Mäck G, Vunkova-Radeva R, Tischner R (1996) Changes in nitrate reductase activity and the protective effect of molybdenum during cold stress in winter wheat grown on acid soil. J Plant Physiol 149:211–221
Yusuf M, Fariduddin Q, Hayat S, Ahmad A (2011) Nickel: an overview of uptake, essentiality and toxicity in plants. Bulletin Environ Contam Toxicol 86:1–17
Zhou B, Guo Z, Xing J, Huang B (2005) Nitric oxide is involved in abscisic acid-induced antioxidant activities in Stylosanthes guianensis. J Exp Bot 56:3223–3228
Author Information
School of Biochemistry, Devi Ahilya University, Indore, India