Establishment of cost-effective rooting, acclimatization and genetic fidelity of in vitro plants of apple rootstock Merton 793
Research Articles | Published: 03 November, 2022
First Page: 1199
Last Page: 1210
Views: 4010
Keywords: Micropropagation, Apple rootstock, In vitro rooting and hardening, Genetic stability, Molecular markers
Abstract
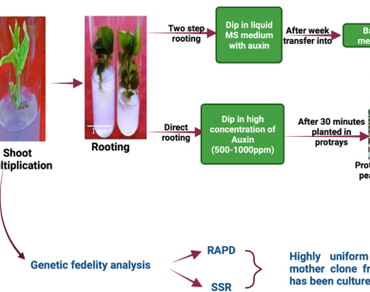
Efforts were made to develop an efficient and low-cost in vitro rooting and hardening of microshoots of Merton 793, an apple rootstock recommended for replantation in the orchards of Himachal Pradesh. Successful rooting of microshoots was related to their exposure to NAA-supplemented MS medium for the first few days and then transferred to the basal medium. Agar was found to be the best substrate among sand, perlite and tapioca pearls. Replacing sucrose with sugar resulted in an equal rooting percentage. Partial in vitro root initiation in a liquid medium and successful root elongation and hardening in peat-sand reduced the duration of the in vitro rooting stage. On the other hand, when shoots were dipped in high levels of NAA and planted for direct rooting and hardening, they eliminated the rooting step. Five-year-old in vitro shoot cultures categorized based on three morphotypes were subjected to random amplified polymorphic DNA and simple sequence repeat analysis. The banding patterns were highly uniform and comparable to the mother clone from which the cultures had been established. Thus, the partial in vitro rooting and direct rooting of shoots of M793 are more economical and can be applied for large-scale production of plants without the risk of instability.
Graphical abstract

References
Alizadeh M, Sing SK (2008) Molecular assessment of clonal fidelity in micropropagated grape (Vitis spp.) rootstock genotypes using RAPD and ISSR markers. Iran J Biotechnol 7:37–44
Balla I, Vertesy J, Vegvari G, Szucs E, Kallay T, Voros I, Biro B (2003) Nutrition of micro-propagated fruit trees in vitro and ex vitro. Int J Hortic Sci 9:43–46
Belaizi M, Sangwan RS, Sangwan-Norreel BS (1989) Control of stages in the micropropagation of apple (Pyrus malus) L. cv. Golden Delicious. Bulletin De La Societie Botanique De France Letters Botaniques 136:11–17
Bennici A, Anzidei M, Vendramin GG (2004) Genetic stability and uniformity of Foeniculum vulgare Mill. regenerated plants through organogenesis and somatic embryogenesis. Plant Sci 166:221–227
Brito G, Lopes T, Loureiro J, Rodriguez E, Santos C (2010) Assessment of genetic stability of two micropropagated wild olive species using flow cytometry and microsatellite markers. Trees 24:723–732
Burg K, Helmersson A, Bozhkov P, Von Arnold S (2007) Developmental and genetic variation in nuclear microsatellite stability during somatic embryogenesis in pine. J Exp Bot 58:687–698
Correa D, De M, Pasqual M, Ishida JS, Alvarenga AA, De Ramos JD (1994) Effects of IBA and mineral salts on in vitro rooting of apple rootstock MI 793 shooots. Revista Ceres 41:379–385
Deverno LL (1995) Evaluation of somaclonal variation during somatic embryogenesis. In: Jain SM, Gupta RK, Newton RJ (eds) Somatic embryogenesis in woody plants: history, molecular biology and biochemical aspects and applications, vol 1. Kluwer Academic Press, Netherlands
Gomez KA, Gomez AA (1984) Statistical procedures for agricultural research. John Wiley and Sons, New York
Gomma AH, Khattah MM, Rahman EA (1989) Effect of rooting media and auxin treatments in apple rootstocks stooling. Egypt J Hortic 16:1–8
Guo WL, Gong L, Ding ZF, Li YD, Li FX, Zhao SP, Liu B (2006) Genomic instability in phenotypically normal regenerants of medicinal plant Codonopsis lanceolate Benth. et Hook. f. as revealed by ISSR and RAPD markers. Plant Cell Rep 25:896–906
Hashmi G, Huettel R, Meyor R, Krusberg L, Hammerschlag F (1997) RAPD analysis of somaclonal variants derived from embryo callus cultures of peach. Plant Cell Rep 16:624–627
Hutchinson JF (1984) Factors affecting shoot proliferation and root initiation in organ cultures of the apple ‘Northern Spy.’ ScientiaHorticulturae 22:347–358
Joshi P, Dhawan V (2007) Assessment of genetic fidelity of micropropagated Swertia Chirayita plantlets by ISSR marker assay. Biol Plant 51:22–26
Khan S, Saeed B, Kauser N (2011) Establishment of genetic fidelity of in-vitro raised banana plantlets. Pak J Bot 43:233–242
Konopelko A (2021) The prognostication of the rooting ability of apple stem cuttings by indices of seasonal growth of shoots. Plant Prod 89–90:101–109
Laszloffy K, Kader AMA, Mathe A (1992) In vitro propagation of ‘JulyRed’ apple. In Vitro Culture 300:149–154
Latoo SK, Bamotra S, Sapru Dhar R, Khan S (2006) Rapid plant regeneration and analysis of genetic fidelity of in vitro derived plants of Chlorophytum arundinaceum baker-an endangered medicinal herb. Plant Cell Rep 26:499–506
Liu YG, Liu LX, Wang L, Gao AY (2008) Determination of genetic stability in surviving apple shoots following cryopreservation by vitrification. CryoLetters 29:7–14
Martelli G, Greco I, Mezzetti B, Rosati P (1993) Isozymic analysis of somaclonal variations among regenerants from apple rootstock leaf tissue. Acta Hortic 336:381–388
Martin KP, Pachathundikandi SK, Zhang CL, Slater A, Madassery J (2006) RAPD analysis of a variant of banana (Musa sp.) cv. grande naine and its propagation via shoot tip culture. In Vitro Cell Dev Biol-Plant 42:188–192
Martins M, Sarmento D, Olivera MM (2004) Genetic stability of micropropagated almond plantlets, as assessed by RAPD and ISSR markers. Plant Cell Rep 23:492–496
Modgil M, Mahajan K, Chakarbarti SK, Sharma DR, Sobti RC (2005) Molecular analysis of genetic stability in micropropagated apple rootstock MM106. Sci Hortic 104:151–160
Modgil M, Sharma T, Thakur M (2009) Commercially feasible protocol for rooting and acclimatization of micropropagated apple rootstocks. Acta Hort 839:209–214
Modgil M, Parmar S, Negi NP (2017) RAPD analysis of long term micropropagated rootstock plants of apple Malling 7. Indian J Exp Biol 55:178–183
Mohan R, Soccol CR, Quoirin M, Pandey A (2004) Use of sugarcane bagasse as an alternative low-cost support material during the rooting stage of apple micropropagation. In Vitro Cell Dev Biol-Plant 40:408–411
Murashige T, Skoog F (1962) A Revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:473–497
Negi NP, Goel A. (2018) Effect of plant growth regulators, salts, activated charcoal and substrates in invitro rooting and acclimatization of Malus domestica. Int J Inf Computing Sci. ISSN NO: 0972-1347
Nene YL, Sheila VK, Moss JP (1996) Tapioca- a potential substitute for agar in the tissue culture media. Curr Sci 70:493–494
Palombi M, Damiano C (2002) Comparision between RAPD and SSR molecular markers in detecting genetic variation in kiwifruit (Actinidia deliciosa A. Chev). Plant Cell Rep 20:1061–1066
Panda MK, Mohanty S, Subudhi E, Acharya L, Nayak S (2007) Assessment of genetic stability of micropropagated plants of Curcuma longa L. by cytophotometry and RAPD analyses. Int J Integerated Biol 1:189–195
Qiang DG, Xin SX, Liang Z, Kun MB (2006) Isozyme patterns and RAPD analysis of apple plantlets repeatedly subcultured in vitro. Acta Hort 33:33–37
Rani V, Raina SN (2000) Genetic fidelity of organized meristem- derived micropropagated plants: a critical reappraisal. In Vitro Cell Dev Biol Plant 36:319–330
Shenoy VB, Vasil IK (1992) Biochemical and molecular analysis of plants derived from embryogenic tissue cultures of napier grass (Pennisetum purpureum K. Schum). Theor Appl Genet 83:947–952
Snir I, Erez A (1983) In vitro propagation of malling merton apple rootstocks. HortScience 15:597–598
Soni M, Thakur M, Modgil M (2011) In vitro multiplication of Merton 793-an apple rootstock suitable for replantation. Indian J Biotechnol 10:362–368
Author Information
University Institute of Biotechnology, Chandigarh University, Mohali, India