Evaluation and characterization of EMS induced mutant population of Gossypium herbaceum
Research Articles | Published: 03 May, 2022
First Page: 1036
Last Page: 1046
Views: 3513
Keywords: Agro-morphological traits, EMS, Gossypium herbaceum , Genetic diversity, RAPD
Abstract
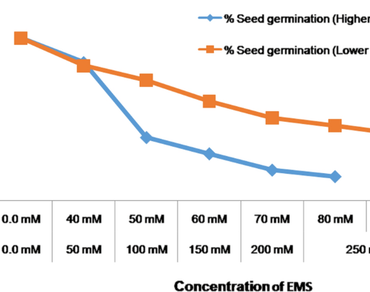
Gossypium herbaceum is reported to have innate adaptability to withstand biotic and abiotic stresses. However, the continued selection for desired traits eliminates the rare alleles from advanced cultivars and makes the species vulnerable to several stresses. The induced mutagenesis expands the genetic basis of the existing germplasm. In the present study, 5500 seeds of G. herbaceum (cv. WAGAD) were treated by 70 mM ethyl methane sulfonate (EMS), established 2597 fertile M1 plants, which were used in development of 6600 M2 plants followed by 5473 M3 plants. About 5% of the plants showed visible mutant phenotypes like sterility, chlorosis, altered leaf morphology and early flowering/late flowering. The phenotypic evaluation of 4453 plants for 11 agro-morphological traits revealed significant variations. Random amplified polymorphic DNA (RAPD) profiling of a random set of 150 M3 plants revealed the considerable level of mutation in the genome. The developed mutant population could serve as an important genetic resource for forward and reverse genetic studies. Further, the potential mutant plants could also be used for pyramiding targeted traits and consequently for developing new high yielding varieties.
References
Abid MA, Wang P, Zhu T, Liang C, Meng Z, Malik W, Guo S, Zhang R (2020) Construction of Gossypium barbadense mutant library provides genetic resources for cotton germplasm improvement. Int J Mol Sci 21(18):6505. https://doi.org/10.3390/ijms21186505
Aslam U, Cheema H, Ahmad S, Khan IA, Malik W, Khan AA (2016) COTIP: cotton TILLING platform, a resource for plant improvement and reverse genetic studies. Front Plant Sci 7:1863. https://doi.org/10.3389/fpls.2016.01863
Aslam R, Bhat TM, Choudhary S, Ansari MYK, Shahwar D (2017) Estimation of genetic variability, mutagenic effectiveness and efficiency in M2 flower mutant lines of Capsicum annuum L. treated with caffeine and their analysis through RAPD markers. J King Saud Univ Sci 29(3):274–283. https://doi.org/10.1016/j.jksus.2016.04.008
Bechere E, Auld DL, Dotray P, Kebede H (2010) Registration of four upland cotton (Gossypium hirsutum L.) genetic stock mutants with tolerance to Imazamox. J Plant Regist 4(2):155–158. https://doi.org/10.3198/jpr2009.08.0446crgs
Brown N, Smith CW, Hague S, Auld D, Hequet E, Joy K, Jones D (2015) Within-boll yield characteristics and their correlation with fiber quality parameters following mutagenesis of upland cotton, TAM 94L–25. Crop Sci 55(4):1513–1523. https://doi.org/10.2135/cropsci2014.06.0442
Chantreau M, Grec S, Gutierrez L, Dalmais M, Pineau C, Demailly H, Paysant-Leroux C, Tavernier R, Trouve JP, Chatterjee M, Guillot X, Brunaud V, Chabbert B, Wuytswinkel O, Bendahmane A, Thomasset B, Hawkins S (2013) PT-Flax (phenotyping and TILLING of flax): development of a flax (Linum usitatissimum L.) mutant population and TILLING platform for forward and reverse genetics. BMC Plant Biol 13(1):159. https://doi.org/10.1186/1471-2229-13-159
Chawade A, Sikora P, Bräutigam M, Larsson M, Vivekanand V, Nakash MA, Chen T, Olsson O (2010) Development and characterization of an oat TILLING population and identification of mutations in lignin and beta glucan biosynthesis genes. BMC Plant Biol 10:86. https://doi.org/10.1186/1471-2229-10-86
Chen L, Huang L, Min D, Phillips A, Wang S, Madgwick PJ, Parry MAJ, Hu YG (2012) Development and characterization of a new TILLING population of common bread wheat (Triticum aestivum L.). PLoS One 7(7):e41570. https://doi.org/10.1371/journal.pone.0041570
Dahmani-Mardas F, Troadec C, Boualem A, LeveˆQue S, Alsadon AA, Aldoss AA, Dogimont C, Bendahmane A (2010) Engineering melon plants with improved fruit shelf life using the TILLING approach. PLoS ONE 5(12):e15776. https://doi.org/10.1371/journal.pone.0015776
Danylchenko O, Sorochinsky B (2005) Use of RAPD assay for the detection of mutation changes in plant DNA induced by UV-B and γ-rays. BMC Plant Biol 5:S9. https://doi.org/10.1186/1471-2229-5-S1-S9
Dhakshanamoorthy D, Selvaraj R, Chidambaram A (2013) Induced mutagenesis in Jatropha curcas L. using Ethyl methanesulphonate (EMS) and assessment of DNA polymorphism through RAPD markers. J Crop Sci Biotechnol 16(3):201–207. https://doi.org/10.1016/j.crvi.2010.11.004
Espina MJ, Ahmed C, Bernardini A, Adeleke E, Yadegari Z, Arelli P, Pantalone V, Taheri A (2018) Development and phenotypic screening of an Ethyl methanesulfonate mutant population in soybean. Front Plant Sci 9:394. https://doi.org/10.3389/fpls.2018.00394
Gunapati S, Naresh R, Ranjan S, Nigam D, Hans A, Verma PC, Gadre R, Pathre UV, Sane AP, Sane VA (2016) Expression of GhNAC2 from G. herbaceum, improves root growth and imparts tolerance to drought in transgenic cotton and Arabidopsis. Sci Rep 6:24978. https://doi.org/10.1038/srep24978
Hammer Ø, Harper D, Ryan P (2001) PAST: Paleontological statistics software package for education and data analysis. Palaeontol Electron 4(1):9
Hasan N, Choudhry S, Laskar RA (2020) Studies on qualitative and quantitative characters of mutagenised chili populations induced through MMS and EMS. Vegetos 33(4):793–799. https://doi.org/10.1007/s42535-020-00164-z
Jena SN, Srivastava A, Rai KM, Ranjan A, Singh SK, Nisar T, Srivastava M, Bag SK, Mantri S, Asif MH, Yadav HK, Tuli R, Sawant SV (2012) Development and characterization of genomic and expressed SSRs for levant cotton (Gossypium herbaceum L.). Theor Appl Genet 124(3):565–576. https://doi.org/10.1007/s00122-011-1729-y
Khan A, Pan X, Najeeb U, Tan DKY, Fahad S, Zahoor R, Luo H (2018) Coping with drought: stress and adaptive mechanisms, and management through cultural and molecular alternatives in cotton as vital constituents for plant stress resilience and fitness. Biol Res. https://doi.org/10.1186/s40659-018-0198-z
Krupa-Małkiewicz M, Bienias A (2018) BSA and molecular markers screening for salt stress tolerant mutant of Petunia obtained in in vitro culture. Ciência Rural. https://doi.org/10.1590/0103-8478cr20170042
Kulkarni VN, Khadi BM, Maralappanavar MS, Deshapande LA, Narayanan S (2009) The worldwide gene pools of Gossypium arboreum L. and G. herbaceum L., and their improvement. Genetics and Genomics of Cotton pp 69–97. https://doi.org/10.1007/978-0-387-70810-2_4
Kumar AP, Boualem A, Bhattacharya A, Parikh S, Desai N, Zambelli A, Leon A, Chatterjee M, Bendahmane A (2013) SMART–Sunflower mutant population and reverse genetic tool for crop improvement. BMC Plant Biol 13:38. https://doi.org/10.1186/1471-2229-13-38
Kumar U, Sawant SV, Yadav HK (2021) Exploring genetic variability in Ethyl methanesulfonate mediated mutant population of Wagad cultivar of Gossypium herbaceum. J Environ Biol 42(3):589–596. https://doi.org/10.22438/jeb/42/3/MRN-1399
Kurowska M, Daszkowska-Golec A, Gruszka D, Marzec M, Szurman M, Szarejko I, Maluszynski M (2011) TILLING-a shortcut in functional genomics. J Appl Genet 52(4):371–390. https://doi.org/10.1007/s13353-011-0061-1
Laskar RA, Khan S (2017) Assessment on induced genetic variability and divergence in the mutagenized lentil populations of microsperma and macrosperma cultivars developed using physical and chemical mutagenesis. PLoS ONE 12(9):e0184598. https://doi.org/10.1371/journal.pone.0184598
Lian X, Liu Y, Guo H, Fan Y, Wu J, Guo H, Gou Z (2020) Ethyl methanesulfonate mutant library construction in Gossypium hirsutum L. for allotetraploid functional genomics and germplasm innovation. Plant J 103(2):858–868. https://doi.org/10.1111/tpj.14755
Maghuly F, Pabinger S, Krainer J, Laimer M (2018) The pattern and distribution of induced mutations in J. curcas using reduced representation sequencing. Front Plant Sci 9:524. https://doi.org/10.3389/fpls.2018.00524
Mardas FD, Troadec C, Boualem A, Leveque S, Alsadon AA, Aldoss AA, Dogimont C, Bendahmane A (2010) Engineering melon plants with improved fruit shelf life using the TILLING approach. PLoS ONE 5(12):e15776. https://doi.org/10.1371/journal.pone.0015776
Parekh MJ, Kumar S, Zala HN, Fougat RS, Patel CB, Bosamia TC, Kulkarni KS, Parihar A (2016) Development and validation of novel fiber relevant dbEST-SSR markers and their utility in revealing genetic diversity in diploid cotton (Gossypium herbaceum and G. arboreum). Ind Crops Prod 83:620–629. https://doi.org/10.1016/j.indcrop.2015.12.061
Parekh MJ, Kumar S, Fougat RS, Zala HN, Pandit RJ (2018) Transcriptomic profiling of developing fiber in levant cotton (Gossypium herbaceum L.). Funct and Integr Genomics 18(2):211–223. https://doi.org/10.1007/s10142-017-0586-4
Perrier X, Flori A, Bonnot F (2003) Data analysis methods. In: Hamon P, Seguin M, Perrier X, Glaszmann JC (eds) Genetic diversity of cultivated tropical plants. Enfield Science Publishers, Montpellier, pp 43–76
Raj A, Kumar S, Haq I, Kumar M (2014) Detection of tannery effluents induced DNA damage in mung bean by use of random amplified polymorphic DNA markers. ISRN Biotechnol. https://doi.org/10.1155/2014/727623
Ranjan A, Nigam D, Asif MH, Singh R, Ranjan S, Mantri S, Pandey N, Trivedi I, Rai KM, Jena SN, Koul B, Tuli R, Pathre UV, Sawant SV (2012) Genome wide expression profiling of two accession of G. herbaceum L. in response to drought. BMC genom 13:94. doi: https://doi.org/10.1186/1471-2164-13-94
Rawat N, Schoen A, Singh L, Mahlandt A, Wilson DL, Liu S, Lin G et al (2018) TILL-D: An Aegilops tauschii TILLING resource for wheat improvement. Front Plant Sci 9:1665. https://doi.org/10.3389/fpls.2018.01665
Sabetta W, Alba V, Blanco A, Montemurro C (2011) sunTILL: a TILLING resource for gene function analysis in sunflower. Plant Methods 7:20. https://doi.org/10.1186/1746-4811-7-20
Saito T, Ariizumi T, Okabe Y, Asamizu E, Hiwasa-Tanase K, Fukuda N, Mizoguchi T, Yamazaki Y, Aoki K, Ezura H (2011) TOMATOMA: a novel tomato mutant database distributing Micro-Tom mutant collections. Plant Cell Physiol 52(2):283–296. https://doi.org/10.1093/pcp/pcr004
Schreiber M, Barakate A, Uzrek N, Macaulay M, Sourdille A, Morris J, Hedley PE, Ramsay L, Waugh R (2019) A highly mutagenised barley (cv. Golden Promise) TILLING population coupled with strategies for screening-by-sequencing. Plant Methods 15(1):99. https://doi.org/10.1186/s13007-019-0486-9
Slade AJ, Mcguire C, Loeffler D, Mullenberg J, Skinner W, Fazio G, Holm A, Brandt KM, Steine MN, Goodstal JF (2012) Development of high amylose wheat through TILLING. BMC Plant Biol 12(10):1186. https://doi.org/10.1186/1471-2229-12-69
Taryono T, Cahyaningrum P (2011) The detection of mutational changes in Sorghum using RAPD. Indones J Biotechnol 16(1)
Tiliouine WA, Laouar M, Abdelguerfi A, Cieslak JJ, Jankuloski L, Till BJ (2018) Genetic variability induced by gamma rays and preliminary results of low-cost TILLING on M2 generation of chickpea (Cicer arietinum L.). Front Plant Sci 9:156. https://doi.org/10.3389/fpls.2018.01568
Trivedi I, Ranjan A, Sharma YK, Sawant S (2012) The histone H1 variant accumulates in response to water stress in the drought tolerant genotype of Gossypium herbaceum L. Protein J 31(6):477–486. https://doi.org/10.1007/s10930-012-9425-6
Wang M, Wang Q, Wang B (2012) Identification and characterization of microRNAs in Asiatic cotton (Gossypium arboreum L.). PLoS One 7(4):e33696. https://doi.org/10.1371/journal.pone.0033696
Yunus MF, Aziz MA, Kadir MA, Daud SK, Rashid AB (2013) In vitro mutagenesis of Etlingera elatior (Jack) and early detection of mutation using RAPD markers. Turk J Biol 37:716–725. https://doi.org/10.3906/biy-1303-19
Author Information
Plant Genetic Resources and Improvement, CSIR-National Botanical Research Institute, Rana Pratap Marg, Lucknow, India