Inter simple sequence repeat (ISSR) markers reveal DNA stability in pineapple plantlets after shoot tip cryopreservation
Villalobos-Olivera Ariel, Ferreira Claudia Fortes, Yanes-Paz Ermis, Lorente Gustavo Y., Souza Fernanda Vidigal, Engelmann Florent, Martínez-Montero Marcos Edel, Lorenzo José Carlos
Research Articles | Published: 14 January, 2022
First Page: 360
Last Page: 366
Views: 3956
Keywords: Ananas comosus , Cryopreservation, Ex situ conservation, Genetic stability, Molecular markers
Abstract
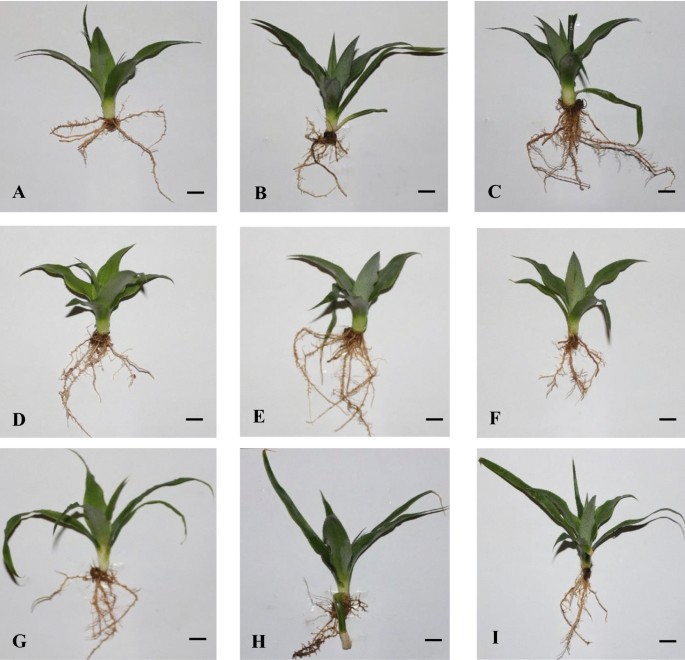
Although pineapple (Ananas comosus var. comosus) shoot tips have been cryopreserved but the possible effect of this process at the molecular level has not been studied. This communication describes the growth (plant fresh and dry weights; stem height; leaf length, width and area; and stem base diameter) and the Inter Simple Sequence Repeat (ISSR) analysis of pineapple plantlets of A. comosus MD-2; Red Spanish Florencia; and Hybrid 54 (Smooth Cayenne/Red Spanish) after 45 d of acclimatization. From each of these varieties, the acclimatized plants were obtained from: (1) conventional micropropagation (control 1); (2) from shoot tips submitted to pre-cryostorage conditioning treatments but not exposed to liquid nitrogen (LN) (treatment 2); and (3) from shoot tips exposed to cryostorage including use of LN (treatment 3). The ISSR-PCR method was used to study the genetic stability. There were no statistically significant differences between treatments for the phenotype indicators evaluated. On average, 45 day-old pineapple plants had 0.5 g fresh weight; 1.85 g dry weight; 12.2 cm stem height; 9.1 cm leaf length; 1.6 cm leaf width; 7.1 cm2 leaf area; and 1.4 cm stem base diameter. Also, the potential effects of cryopreservation at the DNA level were not revealed with the eight ISSR markers used, as no polymorphic bands were recorded, which represents 100% genetic stability. As far as we know, this is the first publication on ISSR analysis of pineapple plantlets after cryopreservation.
References
Acosta Y, Hernández L, Mazorra C, Quintana N, Zevallos BE, Cejas I, Fontes D (2019) Seed cryostorage enhances subsequent plant productivity in the forage species Teramnus labialis (L.F.) Spreng. CryoLetters 40:36–44
Acosta Y, Santiago F, Escalante D, Mazorra C, Cejas I, Martínez-Montero ME, Fontes D (2020) Cryo-exposure of Neonotonia wightii Wigth & Am seeds enhances field performance of plants. Acta Physiol Plant. https://doi.org/10.1007/s11738-019-3010-y
Agrawal A, Sanayaima R, Singh R, Tandon R, Verma S, Tyagi R (2014) Phenotypic and molecular studies for genetic stability assessment of cryopreserved banana meristems derived from field and in vitro explant sources. In Vitro Cell Dev Biol Biol Plant 50:345–356. https://doi.org/10.1007/s11627-014-9606-4
Aragón C, Pascual P, González J, Escalona M, Carvalho L, Amancio S (2013) The physiology of ex vitro pineapple (Ananas comosus L. Merr. var MD-2̕′) as CAM or C3 is regulated by the environmental conditions: proteomic and transcriptomic profiles. Plant Cell Rep 32:1807–1818. https://doi.org/10.1007/s00299-013-1493-3
Atmakuri AR, Chaudhury R, Malik S, Kumar S, Ramachandran R, Qadri S (2009) Mulberry biodiversity conservation through cryopreservation. In Vitro Cell Dev Biol 45:639. https://doi.org/10.1007/s11627-008-9185-3
Carlier JD, Reis A, Duval MF, D’Eeckenbrugge CG, Leitão JM (2004) Genetic maps of RAPD, AFLP and ISSR markers in Ananas bracteatus and A. comosus using the pseudo-testcross strategy. Plant Breed 123:186–192. https://doi.org/10.1046/J.1439-0523.2003.00924.X
Channuntapipat C, Sedgley M, Collins G (2003) Changes in methylation and structure of DNA from almond tissues during in vitro culture and cryopreservation. J Am Soc Hortic Sci 128:890–897. https://doi.org/10.21273/JASHS.128.6.0890
Chen H, Hu B, Zhao L, Shi D, She Z, Huang X, Qin Y (2019) Differential expression analysis of reference genes in pineapple (Ananas comosus L.) during reproductive development and response to abiotic stress. Trop Plant Biol 12:1–11. https://doi.org/10.1007/s12042-019-09218-2
da Silva RL, Ferreira CF, da Silva Ledo CA, de Souza EH, da Silva PH, de Carvalho Costa MAP, Souza FVD (2016) Viability and genetic stability of pineapple germplasm after 10 years of in vitro conservation. Plant Cell Tissue Organ Cult 127:123–133
Daquinta M, Benegas R (1997) Brief review of tissue culture of pineapple. Pineap News 3:7–9
DeVerno L, Park Y, Bonga J, Barrett J, Simpson C (1999) Somaclonal variation in cryopreserved embryogenic clones of white spruce [Picea glauca (Moench) Voss.]. Plant Cell Report 18:948–953. https://doi.org/10.1007/s002990050689
Ebel AI, Giménez L, González AM, Alayón Luaces P (2016) Evaluación morfoanatómica de hojas" D" de piña (Ananas comosus (L.) Merr.) en respuesta a la implantación de dos sistemas de cultivo en Corrientes. Acta Agron 65:390–397. https://doi.org/10.15446/ACAG.V65N4.50560
Engelmann F, Ramanatha R (2012) Major research challenges and directions for future research. In: Normah MN, Chin HF, Reed BM (eds) Conservation of tropical plant species. Springer, Berlin, pp 511–526. https://doi.org/10.1007/978-1-4614-3776-5_20
Espasandin FD, Brugnoli EA, Ayala PG, Ayala LP, Ruiz OA, Sansberro PA (2019) Long-term preservation of Lotus tenuis adventitious buds. Plant Cell Tissue Organ Cult 136:373–382. https://doi.org/10.1007/s11240-018-1522-6
FAOSTAT. (2021) FAO Statistics Division [online]. Disponible. http://faostat.fao.org/site/567/. Accessed 9 Oct 2021
Gómez D, Hernández L, Valle B, Martínez J, Cid M, Escalona M, Lorenzo JC (2017) Temporary immersion bioreactors (TIB) provide a versatile, cost-effective and reproducible in vitro analysis of the response of pineapple shoots to salinity and drought. Acta Physiol Plant 39:277
Johnston JW, Benson EE, Harding K (2009) Cryopreservation induces temporal DNA methylation epigenetic changes and differential transcriptional activity in Ribes germplasm. Plant Physiol Biochem 47:123–131. https://doi.org/10.1016/j.plaphy.2008.10.008
Kaity A, Ashmore S, Drew R, Dulloo M (2008) Assessment of genetic and epigenetic changes following cryopreservation in papaya. Plant Cell Report 27:1529–1539. https://doi.org/10.1007/s00299-008-0558-1
Kaya E (2016) ISSR analysis for determination of genetic diversity and relationship in some Turkish olive (Olea europaea L) cultivars. Not Bot Hortic Agrobot Lujapoca 43:96–99. https://doi.org/10.15835/nbha4319818
Kaya E, Souza FVD (2017) Comparison of two PVS2-based procedures for cryopreservation of commercial sugarcane (Saccharum spp.) germplasm and confirmation of genetic stability after cryopreservation using ISSR markers. In Vitro Cell Dev Biol Plant 53:410–417. https://doi.org/10.1007/s11627-017-9837-2
Kobayashi T, Heck DJ, Nomura M, Horiuchi T (1998) Expansion and contraction of ribosomal DNA repeats in Saccharomyces cerevisiae: requirement of replication fork blocking (Fob1) protein and the role of RNA polymerase I. Genes Dev 12:3821–3830. https://doi.org/10.1101/gad.12.24.3821
Lambardi M, De Carlo A, Biricolti S, Puglia A, Lombardo G, Siragusa M, De Pasquale F (2004) Zygotic and nucellar embryo survival following dehydration/cryopreservation of Citrus intact seeds. CryoLetters 25:81–90
Liu Y-G, Liu L-X, Wang L, Gao A-Y (2008) Determination of genetic stability in surviving apple shoots following cryopreservation by vitrification. CryoLetters 29:7–14
Martínez-Montero M, Engelmann F, González-Arnao M (2012) Cryopreservation of tropical plant germplasm with vegetative propagation-review of sugarcane (Saccharum spp.) and pineapple (Ananas comusus (L.) Merrill) cases. In: Katkov I (ed) Current frontiers in cryopreservation. London England, pp 260–396. https://doi.org/10.5772/32047
Matsumoto T, Yamamoto S, Fukui K, Rafique T, Engelmann F, Niino T (2015) Cryopreservation of persimmon shoot tips from dormant buds using the D cryo-plate technique. Hortic J 84:106–110. https://doi.org/10.2503/HORTJ.MI-043
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue culture. Physiol Plant 5:473–497. https://doi.org/10.1111/j.1399-3054.1962.tb08052.x
Nalousi AM, Hatamzadeh A, Azadi P, Mohsenpour M, Lahiji HS (2019) A procedure for indirect shoot organogenesis of Polianthes tuberosa L. and analysis of genetic stability using ISSR markers in regenerated plants. Sci Hortic 244:315–321. https://doi.org/10.1016/J.SCIENTA.2018.09.066
Pino Y, Concepción O, Santos R, González J, Rodríguez R (2014) Effect of Previcur® energy fungicide on MD-2 pineapple (Ananas comosus var. comosus) plantlets during the acclimatization phase. Pineapple News 1:1–24
Rameshkumar R, Pandian S, Rathinapriya P, Selvi CT, Satish L, Gowrishankar S, Ramesh M (2019) Genetic diversity and phylogenetic relationship of Nilgirianthus ciliatus populations using ISSR and RAPD markers: implications for conservation of an endemic and vulnerable medicinal plant. Biocatal Agric Biotechnol 18:101072. https://doi.org/10.1016/J.BCAB.2019.101072
Rao AA, Chaudhury R, Kumar S, Velu D, Saraswat R, Kamble C (2007) Cryopreservation of mulberry germplasm core collection and assessment of genetic stability through ISSR markers. Int J Ind Entomol 15:23–33
Reddy MP, Sarla N, Siddiq E (2002) Inter simple sequence repeat (ISSR) polymorphism and its application in plant breeding. Euphytica 128:9–17. https://doi.org/10.1023/A%3A1020691618797
Shingote PR, Mithra SA, Sharma P, Devanna NB, Arora K, Holkar SK, Sharma T (2019) LTR retrotransposons and highly informative ISSRs in combination are potential markers for genetic fidelity testing of tissue culture-raised plants in sugarcane. Mol Breed 39:25. https://doi.org/10.1007/s11032-019-0931-5
Silva JM, Lima PR, Souza FV, Ledo CA, Souza EH, Pestana KN, Ferreira CF (2019) Genetic diversity and nonparametric statistics to identify possible ISSR marker association with fiber quality of pineapple. An Acad Bras Ciênc 91:144–154
Souza F, Kaya E, de Jesus VL, de Souza E, de Oliveira AV, Skogerboe D, Jenderek M (2015) Droplet-vitrification and morphohistological studies of cryopreserved shoot tips of cultivated and wild pineapple genotypes. Plant Cell Tissue Organ Cult 124:351–360. https://doi.org/10.1007/s11240-015-0899-8
Souza CPF, Ferreira CF, de Souza EH, Neto ARS, Marconcini JM, da Silva Ledo CA, Souza FVD (2017) Genetic diversity and ISSR marker association with the quality of pineapple fiber for use in industry. Ind Crops Prod 104:263–268
Souza FVD, de Souza EH, Kaya E, de Jesus VL, da Silva RL (2018) Cryopreservation of pineapple shoot tips by the droplet vitrification technique. Plant Cell Cult Protocols 124:269–277. https://doi.org/10.1007/s11240-015-0899-8
Tapia C, Gutiérrez G, Warbourton L, Uriza A, Rebolledo M (2002) Characterization of pineapple germplasm (Ananas spp.) by mean AFLPs. In: IV international pineapple symposium 666, pp 109–114
Vanijajiva O (2012) Assessment of genetic diversity and relationships in pineapple cultivars from Thailand using ISSR marker. J Agric Technol 8:1829–1838
Villalobos A, González J, Santos R, Rodríguez R (2012) Morpho-physiological changes in pineapple plantlets (Ananas comosus (L.) Merr.) during acclimatization. Ciência e Agrotecnol 36:624–630. https://doi.org/10.1590/S1413-70542012000600004
Villalobos-Olivera A, Martínez J, Quintana N, Zevallos BE, Cejas I, Lorenzo JC, Montero MEM (2019) Field performance of micropropagated and cryopreserved shoot tips-derived pineapple plants grown in the field for 14 months. Acta Physiol Plant 41:34. https://doi.org/10.1007/s11738-019-2825-x
Wang B, Li JW, Zhang ZB, Wang RR, Ma YL, Blystad DR, Wang QC (2014a) Three vitrification-based cryopreservation procedures cause different cryo-injuries to potato shoot tips while all maintain genetic integrity in regenerants. J Biotechnol 184:47–55. https://doi.org/10.1016/j.jbiotec.2014.04.021
Wang B, Wang RR, Cui ZH, Bi WL, Li JW, Li BQ, Wang QC (2014b) Potential applications of cryogenic technologies to plant genetic improvement and pathogen eradication. Biotechnol Adv 32:583–595. https://doi.org/10.1016/j.biotechadv.2014.03.003
Yabor L, Pérez L, Gómez D, Villalobos-Olivera A, Mendoza JR, Martínez J, Lorenzo JC (2020) Histological evaluation of pineapple transgenic plants following eight years of field growth. Euphytica 216:1–8. https://doi.org/10.1007/s10681-020-2555-6
Yamuna G, Sumathi V, Geetha S, Praveen K, Swapna N, Nirmal Babu K (2007) Cryopreservation of in vitro grown shoots of ginger (Zingiber officinale Rosc.). CryoLetters 28:241–252
Yanes-Paz E, González J, Sánchez R (2000) A technology of acclimatization of pineapple vitroplants. Pineap News 7:24
Yanes-Paz E, Gil K, Rebolledo L, Rebolledo A, Uriza D, Martínez O, Simpson J (2012) Genetic diversity of Cuban pineapple germplasm assessed by AFLP markers. Crop Breed Appl Biotechnol 12:104–110. https://doi.org/10.1590/S1984-70332012000200002
Author Information
University of Ciego de Ávila, Ciego de Ávila, Cuba