Characterization of Camellia chrysantha nano-formulated powder and its cytotoxic activity
*Article not assigned to an issue yet
Hoang Le M., Nguyen Hoa T., Nguyen Phi H., Anh Khuc D. D., Khanh Nguyen C. N., Hoa Truong T. T., Huy Tran Q., To Dao Cuong, Nguyen Hoa T., Nguyen Phi H.
Short Communications | Published: 18 July, 2025
First Page: 0
Last Page: 0
Views: 7
Keywords: n Camellia chrysanthan , n C. chrysanthan , Nano-formulated powder (CNFP), Laser diffraction spectroscopy (LDS), Scanning electron microscopy (SEM), X-ray diffraction (XRD), Transmission electron microscopy (TEM), cytotoxic
Abstract
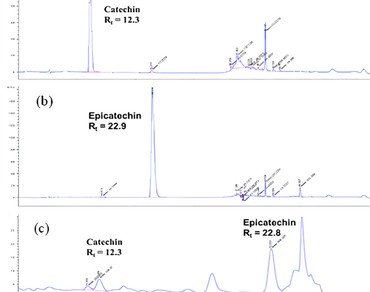
The Golden Camellia, scientifically known as Camellia chrysantha (Hu) Tuyama (Theaceae), is an evergreen shrub native to Southeast and East Asia, known for its diverse health benefits. The ethanolic extract of C. chrysantha leaves, obtained via reflux extraction using 10 L of ethanol, contains various bioactive compounds with potential medical applications. However, its poor water solubility limits its therapeutic application. This study aimed to enhance the bioavailability and cytotoxic efficacy of the extract by synthesizing a nano-formulated powder (CNFP) using a top-down approach. Laser diffraction spectroscopy (LDS) and scanning electron microscopy (SEM) revealed CNFP with a mean particle size of 134 ± 14 nm and a polydispersity index (PdI) of 0.285. Otherwise, X-ray diffraction (XRD) showed that CNFP has an amorphous structure (XRD peak at 2θ ≈ 21°), porous morphology (transmission electron microscopy, TEM), and good colloidal stability (zeta potential 38.5 mV). The cytotoxicity of CNFP was evaluated against four human cancer cell lines—MCF-7 (breast cancer), HepG2 (hepatocellular carcinoma), HL60 (human leukemia), and A549 (lung cancer)—using the 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyl tetrazolium bromide (MTT) assay. CNFP showed stronger cytotoxic activities than C. chrysantha extract (p < 0.05), specifically with IC50 values of 194.12 ± 8.02, 332.07 ± 10.14, 223.83 ± 12.80, and 372.94 ± 18.25 µg/mL, respectively. The enhanced cytotoxicity is attributed to the improved aqueous dispersibility and cellular uptake of the nano-formulated particles. While these results indicate promising in vitro anticancer potential, further in vivo studies are required to validate the efficacy and safety of CNFP for clinical applications.
References
Augustine R, Hasan A, Das S, Ahmed R, Mori Y, Hasan A, Thakor AS (2020) Cancer nanotechnology: the role of nanoparticles in the diagnosis and treatment of cancer. Mater Sci Eng C 106:110056. https://doi.org/10.1016/j.msec.2019.110056
Azad AK, Doolaanea AA, Al-Mahmood SMA, Kennedy JF, Chatterjee B, Bera H (2021) Electro-hydrodynamic assisted synthesis of lecithin-stabilized peppermint oil-loaded alginate microbeads for intestinal drug delivery. Int J Biol Macromol 185(2021):861–875. https://doi.org/10.1016/j.ijbiomac.2021.07.019
Bates S, Zografi G, Engers D, Morris K, Crowley K, Newman A (2006) Analysis of amorphous and nanocrystalline solids from their X-ray diffraction patterns. Pharm Res 23:2333–2349. https://doi.org/10.1007/s11095-006-9086-2
Bhosale PB, Ha SE, Vetrivel P, Kim HH, Kim JA, Park KI, Kim SM, Kim GS (2020) Flavonoid-induced apoptotic cell death in human cancer cells and its mechanisms. J Biomed Transl Res 21(2):50–58. https://doi.org/10.12729/jbtr.2020.21.2.050
Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, Jemal A (2024) Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 74(3):229–263. https://doi.org/10.3322/caac.21834
Danaei M, Dehghankhold M, Ataei S, Hasanzadeh Davarani F, Javanmard R, Dokhani A, Khorasani S, Mozafari MR (2018) Impact of particle size and polydispersity index on the clinical applications of lipidic nanocarrier systems. Pharmaceutics 10(2):57. https://doi.org/10.3390/pharmaceutics10020057
Diep TT (2022) Yellow camellias: A review of chemical constituents and biological activitites. Dalat Univ J Sci 12(3):117–144. https://doi.org/10.37569/DalatUniversity.12.3.977(2022)
Enaru B, Socaci S, Farcas A, Socaciu C, Danciu C, Stanila A, Diaconeasa Z (2021) Novel delivery systems of polyphenols and their potential health benefits. Pharmaceuticals (Basel) 14(10):946. https://doi.org/10.3390/ph14100946
Geetha T, Garg A, Chopra K, Kaur IP (2004) Delineation of antimutagenic activity of catechin, epicatechin and green tea extract. Mutat Res-Fundam Mol Mech Mutagen 556(1–2):65–74. https://doi.org/10.1016/j.mrfmmm.2004.07.003
Honary S, Zahir F (2013) Effect of zeta potential on the properties of nano-drug delivery systems-a review (Part 2). Trop J Pharm Res 12(2):265–273. https://doi.org/10.4314/tjpr.v12i2.20
Jafari SM, Arpagaus C, Cerqueira MA, Samborska K (2021) Nano spray drying of food ingredients; materials, processing and applications. Trends Food Sci Tech 109:632–646. https://doi.org/10.1016/j.tifs.2021.01.061
Lin JN, Lin HY, Yang NS, Li YH, Lee MR, Chuang CH, Ho CT, Kuo SC, Way TD (2023) Chemical constituents and anticancer activity of yellow camellias against MDA-MB-231 human breast cancer cells. J Agric Food Chem 61(40):9638–9644. https://doi.org/10.1021/jf4029877
Luong PH, Nguyen TC, Pham TD, Tran DMT, Ly TNL, Vu QT, Tran TKN, Thai H (2021) Preparation and assessment of some characteristics of nanoparticles based on sodium alginate, chitosan, and Camellia chrysantha polyphenols. Int J Polym Sci 2021(3):5581177. https://doi.org/10.1155/2021/5581177
Mahdavi SA, Jafari SM, Assadpoor E, Dehnad D (2016) Microencapsulation optimization of natural anthocyanins with maltodextrin, gum Arabic and gelatin. Int J Biol Macromol 85:379–385. https://doi.org/10.1016/j.ijbiomac.2016.01.011
Nangare KA, Powar SD, Kate VK, Patwekar SR (2018) Therapeutics applications of nanosuspension in topical/mucosal drug delivery. J Nanomed Res 7(1):1–10. https://doi.org/10.15406/jnmr.2018.07.00170
Okumuş E, Bakkalbaş E, Javidipour I, Meral R, Ceylan Z (2021) A novel coating material: Ellagitannins-loaded maltodextrin and lecithin-based nanomaterials. Food Biosci 42:101158. https://doi.org/10.1016/j.fbio.2021.101158
Patel VR, Agrawal Y (2011) Nanosuspension: an approach to enhance solubility of drugs. J Adv Pharm Technol Res 2(2):81–87. https://doi.org/10.4103/2231-4040.82950
Phan ADT, Adiamo O, Akter S, Netzel ME, Cozzolino D, Sultanbawa Y (2021) Effects of drying methods and maltodextrin on vitamin C and quality of Terminalia Ferdinandiana fruit powder, an emerging Australian functional food ingredient. J Sci Food Agric 101(12):5132–5141. https://doi.org/10.1002/jsfa.11159
Quach H, Le TV, Nguyen TT, Nguyen P, Nguyen CK, Dang LH (2022) Nano-lipids based on ginger oil and lecithin as a potential drug delivery system. Pharmaceutics 14(8):1654. https://doi.org/10.3390/pharmaceutics14081654
S’ari M, Blade H, Cosgrove S, Drummond-Brydson R, Hondow N, Hughes LP, Brown A (2021) Characterization of amorphous solid dispersions and identification of low levels of crystallinity by transmission electron microscopy. Mol Pharm 18(5):1905–1919. https://doi.org/10.1021/acs.molpharmaceut.0c00918
Sanjay ST, Pandey CM (2017) Nanoparticle-induced enhancements in drug solubility and bioavailability: mechanistic insights. J Contr Release 258:74–86. https://doi.org/10.1016/j.jconrel.2017.05.002
Shao S, Li L, Yang G, Li J, Luo C, Gong T (2011) Controlled green tea polyphenols release from electrospun pcl/mwcnts composite nanofibers. Int J Pharm 421(2):310–320. https://doi.org/10.1016/j.ijpharm.2011.09.033
Skehan P, Storeng R, Scudiero D, Monks A, McMahon J, Vistica D, Warren JT, Bokesch H (1990) New colorimetric cytotoxicity assay for anticancer-drug screening. J Natl Cancer Inst 82(3):1107–1112. https://doi.org/10.1093/jnci/82.13.1107
Siccama JW, Pegiou E, Zhang L, Mumm R, Hall RD, Boom RM, Schutyser MAI (2021) Maltodextrin improves physical properties and volatile compound retention of spray-dried asparagus concentrate. LWT 142:111058. https://doi.org/10.1016/j.lwt.2021.111058
Tuyama T (1975) On Theopsis Chrysantha Hu. J Jpn Bot 50:297–299
Uddin MJ, Roni MA (2021) Role of excipients in stabilizing nanoparticle formulations: lecithin and maltodextrin as functional carriers. Adv Drug Deliv Rev 173:267–284. https://doi.org/10.1016/j.addr.2021.04.005
Wang L, Roy D, Lin SS, Yuan ST, Sun L (2017) Hypoglycemic effect of Camellia chrysantha extract on type 2 diabetic mice model. Bangladesh J Pharmacol 12(4):359–363. https://doi.org/10.3329/bjp.v12i4.32995
Wei JB, Li X, Song H, Liang YH, Pan YZ, Ruan JX, Qin X, Chen YX, Nong CL, Su ZH (2015) Characterization and determination of antioxidant components in the leaves of Camellia chrysantha (Hu) Tuyama based on composition-activity relationship approach. J Food Drug Anal 23(1):40–48. https://doi.org/10.1016/j.jfda.2014.02.003
Xiao Z, Xia J, Zhao Q, Niu Y, Zhao D (2022) Maltodextrin as wall material for microcapsules: A review. Carbohydr Polym 298(4):120113. https://doi.org/10.1016/j.carbpol.2022.120113
Zhang HL, Wu QX, Wei X, Qin XM (2020) Pancreatic lipase and cholesterol esterase inhibitory effect of Camellia nitidissima Chi flower extracts in vitro and in vivo. Food Biosci 37(3):100682. https://doi.org/10.1016/j.fbio.2020.100682
Zhou Y, Chen D, Xue G, Yu S, Yuan C, Huang M, Jiang L (2020) Improved therapeutic efficacy of quercetin-loaded polymeric nanoparticles on triple-negative breast cancer by inhibiting uPA. RSC Adv 10(57):34517–34526. https://doi.org/10.1039/D0RA04231E
Author Information
Phenikaa University Nano Institute (PHENA), Phenikaa University, Hanoi, Vietnam