Development of synthetic seeds in Arabica coffee embryos under aseptic and non-aseptic conditions
Short Communications | Published: 19 March, 2022
First Page: 839
Last Page: 849
Views: 3861
Keywords: Calcium alginate, Synthetic seeds, Hydrogels technique, Greenhouse, Growth culture room
Abstract
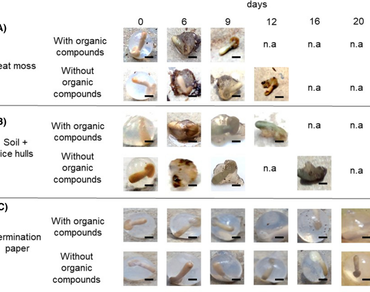
In many tropical countries, Coffea arabica L. is a crop of great commercial and social importance. Synthetic seeds are an effective technique to propagate elite, hybrids, and genetically modified plant material. However, they are highly susceptible to microbial contamination when cultivated in non-aseptic conditions which results in limiting their applications. Here we aimed to evaluate different strategies under both aseptic and non-aseptic conditions to culture encapsulated coffee zygotic embryos with and without organic compounds (vitamins and sucrose). For both methods, encapsulated zygotic embryos were cultured in peat moss, soil with rice husk, and germination paper under greenhouse or plant growth culture room conditions. Growth room conditions resulted in better germination and capsules conservation compared with the greenhouse. The capsules with organic compounds cultivated in germination paper, under growth room conditions and previously sprayed with 1.5 mgl−1 carbendazim, allowed better germination percentages (93%) after 1 month of culture. On the other hand, zygotic embryos encapsulated without organic compounds, under the same conditions, stopped their development and died with the course of the days until obtaining 100% of their mortality.
References
Agresti A (1996) Introduction to categorical data analysis. Wiley, New York
Arcila J, Farfán F, Moreno A et al (2007) Sistemas de Produccion de Café en Colombia. Editorial Blanecolor Ltda, Colombia, p 309
Bekheet SA (2017) Encapsulation of date palm somatic embryos: synthetic seeds. In: Al-Khayri et al (eds) Date palm biotechnology protocols volume II. Methods in molecular biology, vol 1638. Humana Press, New York, pp 71–78. https://doi.org/10.1007/978-1-4939-7159-6_7
Bojórquez-Quintal JEA, Sánchez-Cach LA, Gamboa-Tec NF et al (2011) Effect of plant growth regulators on in vitro germination of coffee zygotic embryos. Afr J Biotech 10(82):19056–19065. https://doi.org/10.5897/AJB11.2794
Cartes P, Castellanos H, Ríos D et al (2009) Encapsulated somatic embryos and zygotic embryos for obtaining artificial seeds of rauli-beech (Nothofagus alpina (Poepp. & Endl.) oerst.). Chil J Agric Res 69:112–118
Chandrasekhara M, Sri K, Pullaiah T (2012) Synthetic seeds: a review in agriculture and forestry. Afr J Biotech 11:14254–14275
Da Rosa SD, Carvalho AM, McDonald MB et al (2011) The effect of storage conditions on coffee seed and seedling quality. Seed Sci Technol 39(1):151–164. https://doi.org/10.15258/sst.2011.39.1.13
Eira M, Da Silva A, De Castro R et al (2006) Coffee seed physiology. Braz J Plant Physiol 18:149–163
Ellis RH, Hong TD, Roberts EH (1991) An intermediate category of seed storage behaviour? Effects of provenance, immaturity, and imbibition on desiccation-tolerance in coffee. J Exp Bot 42(5):653–657. https://doi.org/10.1093/jxb/42.5.653
Etienne H, Breton D, Breitler JC et al (2018) Coffee somatic embryogenesis: how did research, experience gained and innovations promote the commercial propagation of elite clones from the two cultivated species? Front Plant Sci. https://doi.org/10.3389/fpls.2018.01630
George E, Hall A, De Klerk G (2008) Plant propagation by tissue culture, 3rd edn. Springer, Reino Unido, pp 115–173
Hung CD, Dung CD (2015) Production of chrysanthemum synthetic seeds under non-aseptic conditions for direct transfer to commercial greenhouses. Plant Cell Tissue Organ Cult 122:639–648. https://doi.org/10.1007/s11240-015-0797-0
Ibrahim M, Sulistiyorini I (2021) Embryo culture of three coffee species on various fruit ages and media formulations. J Tanaman Ind Dan Penyegar 8(3):151–164. https://doi.org/10.21082/jtidp.v8n3.2021.p151-164
ICAFE (2021) Informes de la actividad cafetalera. http://www.icafe.cr/sector-cafetalero/informacion-de-mercado/informes-de-la-actividad-cafetalera/ Accessed 27 Jan 2022
Islam MS, Bari MA (2012) In vitro regeneration protocol for artificial seed production in an important medicinal plant Mentha arvensis L. J Bio-Sci 20:99–108
Ivamoto ST, Reis O, Domingues DS et al (2017) Transcriptome analysis of leaves, flowers and fruits perisperm of Coffea arabica L reveals the differential expression of genes involved in raffinose biosynthesis. PLoS ONE 12:e0169595
Jung SJ, Yoon ES, Jeong J et al (2004) Enhanced post-germinative growth of encapsulated somatic embryos of Siberian ginseng by carbohydrate addition to the encapsulation matrix. Plant Cell Rep 23(6):365–370. https://doi.org/10.1007/s00299-004-0821-z
Kenneth J (1984) Chloroplasts. Springer Science & Business Media, New York, p 252
Kumar V, Madhava M, Ravishankar GA (2006) Developments in coffee biotechnology—in vitro plant propagation and crop improvement. Plant Cell Tissue Organ Cult 87(1):49–65. https://doi.org/10.1007/s11240-006-9134-y
Lata H, Chandra S, Khan IA et al (2009) Propagation through alginate encapsulation of axillary buds of Cannabis sativa L.—an important medicinal plant. Physiol Mol Biol Plants 15(1):79–86. https://doi.org/10.1007/s12298-009-0008-8
Masaguer A, López-Cuadrado M (2006) Sustratos para viveros. http://www.horticom.com/revistasonline/revistas/viveros06/m_cruz_a_masaguer.pdf. Accessed 25th Feb 2016
Meyer D, Zeileis A, Hornik K (2021). vcd: Visualizing Categorical Data. R package version 1.4–9
Patil I (2021) Visualizations with statistical details: the “ggstatsplot” approach. J Open Source Softw 6(61):3167. https://doi.org/10.21105/joss
Pire R, Pereira A (2003) Propiedades físicas de componentes de sustratos de uso Común en la horticultura del Estado Lara, Venezuela: Propuesta Metodológica. Bioagro 15:55–63
Ravi D, Anand P (2012) Production and applications of artificial seeds: a review. Res J Bio Sci 1(5):74–78
Rihan H, Kareem F, El-Mahrouk M et al (2017) Artificial seeds (Principle, aspects and applications). Agronomy 7(4):71. https://doi.org/10.3390/agronomy7040071
Rosna T, Noraini M, Jamilah Y et al (2013) Synthetic seeds production and regeneration of oxalis triangularis for mass propagation and conservation. Int J Environ Sci Dev 4:461–464
Roy B, Tulsiram SD (2013) Synthetic seed of rice: an emerging avenue of applied biotechnology. Rice Genom Genet. https://doi.org/10.5376/rgg.2013.04.0004
Shah MI, Sultan P, Nasier A et al (2006) In vitro study on effect of some fungicides viz., Carbendazim, Mancozeb, conjoint Carbendazim Mancozeb and sulphur against F. oxysporum. Res J Microbiol 1:360–365
Srivastava S, Singh VP, Kumar R et al (2011) In vitro evaluation of Carbendazim 50% WP, antagonists and botanicals against Fusarium oxysporum f. sp. psidii associated with rhizosphere soil of Guava. Asian J Plant Pathol 5:46–53
Taha RM, Mahmad N, Yaacob JS, Abdullah N, Mohajer S (2013) Synthetic seeds production and regeneration of Oxalis triangularis for mass propagation and conservation. Int J Environ Sci Dev 4:461–464
Author Information
Escuela de Biología, Laboratorio de Biotecnología de Plantas, San Jose Costa Rica, Universidad de Costa Rica, San José, Costa Rica