Evaluation of major anti-nutritional factors in oilseed Brassica
Kumar M. S. Sujith, Mawlong Ibandalin, Kumar Arun, Singh K. H., Gurung Bishal, Rani Reema, Rai P. K.
Research Articles | Published: 15 July, 2022
First Page: 591
Last Page: 598
Views: 3703
Keywords: Total glucosinolates, Phytic acid, Crude fiber, Erucic acid
Abstract
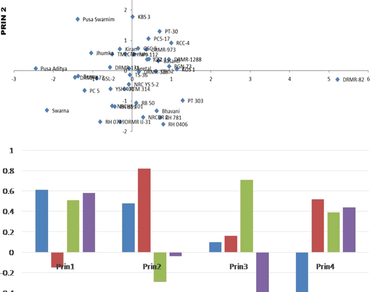
Forty five cultivated varieties of oilseed Brassica belonging to 7 different taxa were analyzed for major antinutritional factors glucosinolates, phytic acid and crude fiber content in seed meal fraction and erucic acid in oil fraction. The glucosinolate content was found to be highest in the Brassica rapa var. Toria group (172.80 μmol/g) with highest level recorded by variety PT 30 (204.77 μmol/g). Varieties belonging to the Eruca sativa (119.05 μmol/g) group were found to have the low glucosinolate content with Brassica carinata variety Pusa Aditya (69.74 μmol/g) recording lowest level. Phytate content was high in the Brassica carinata group (3.31%) with highest level observed in Ragini (6.15%) which is a Yellow Sarson variety. Brassica juncea group (1.2%) showed very low levels of phytate with lowest level found in variety RH 781 (0.2%). Average crude fiber content was highest in Brown sarson group (10.98%) with highest crude fiber content observed in Brassica juncea variety RH0406 (13.39%). The yellow sarson group showed low crude fiber content among all the species with lowest content observed in the variety Jhumka (6.43%). The yellow sarson group exhibited the highest average erucic acid content (40.33%) among all the species with highest level in variety YSH 401 (48.51%). The Brassica napus group showed lowest average erucic acid content (20.71%) with lowest level in variety Neelam (14.12%). The variation in these factors may help to identify potential sources for quality breeding programmes.
References
Alemayehu N, Becker H (2002) Genotypic diversity and patterns of variation in a germplasm material of Ethiopian mustard (Brassica carinata A. Braun). Genet Resour Crop Evol 49(6):573–82
Ali N, Bakht J, Rabbani MA, Khan A (2015) Estimation of variability among indigenous Brassica juncea L. accessions based on morphological and biochemical characteristics. Pak J Agric Sci 1:52(1)
Bala M, Singh M (2013) Non destructive estimation of total phenol and crude fiber content in intact seeds of rapeseed–mustard using FTNIR. Ind Crops Prod 42:357–362
Beare-Rogers JL, Nera EA, Heggtveit HA (1974) Myocardial alteration in rats fed rapeseed oils containing high or low levels of erucic acid. Ann Nutr Metab 17(4):213–222
Bell JM, Keith MO (1991) A survey of variation in the chemical composition of commercial canola meal produced in Western Canadian crushing plants. Can J Anim Sci 71(2):469–480
Chauhan JS, Tyagi MK, Kumar PR, Tyagi P, Singh M, Kumar S (2002) Breeding for oil and seed meal quality in rapeseed-mustard in India–a review. Agric Rev 23(2):71–92
Chauhan JS, Bhadauria VP, Singh M, Singh KH, Kumar A (2007) Quality characteristics and their interrelationships in Indian rapeseed-mustard (Brassica sp) varieties. Indian J Agric Sci 77(9)
Chauhan JS, Kumar S, Singh KH, Meena SS, Meena ML (2010) Oil and seed meal quality indices of Indian rapeseed-mustard varieties. J Plant Biochem Biotechnol 19(1):83–86
Davik J, Heneen WK (1993) Identification of oilseed turnip (Brassica rapa L var oleifera) cultivar groups by their fatty acid and glucosinolate profiles. J Sci Food Agric 63(4):385–390
Deshpande SS, Cheryan M (1984) Effects of phytic acid, divalent cations, and their interactions on α-amylase activity. J Food Sci 49(2):516–519
Erdman JW (1979) Oilseed phytates: nutritional implications. J Am Oil Chem Soc 56(8):736–741
Fenwick GR, Heaney RK, Mawson R (1989) Glucosinolates. In: Cheeke RR (ed) Toxicants of Plant Origin, vol 2. CRC Press, pp 1–41
Font R, Del Río M, Fernández-Martínez JM, de Haro-Bailón A (2004) Use of near-infrared spectroscopy for screening the individual and total glucosinolate contents in Indian mustard seed (Brassica juncea L. Czern. & Coss.). J Agric Food Chem 52(11):3563–9
Grases F, Simonet BM, Prieto RM, March JG (2001) Phytate levels in diverse rat tissues: influence of dietary phytate. Br J Nutr 86(2):225–231
Guttieri M, Bowen D, Dorsch JA, Raboy V, Souza E (2004) Identification and characterization of a low phytic acid wheat. Crop Sci 44:418–424
Haug W, Lantzsch HJ (1983) Sensitive method for the rapid determination of phytate in cereals and cereal products. J Sci Food Agric 34(12):1423–1426
Kaushik N, Sarkar G, Agnihotri A (2007) Variability of glucosinolates in Brassica germplasmcollections.In第十二届国际油菜大会 (The 12th International Rapeseed Congress) (pp. 371–373).华中农业大学.
Khattab R, Goldberg E, Lin L, Thiyam U (2010) Quantitative analysis and free-radical-scavenging activity of chlorophyll, phytic acid, and condensed tannins in canola. Food Chem 122(4):1266–1272
Krzymanski J (1970) Genetic possibilities of improving the chemical composition of winter swede rape seeds. Hodowla Roslin, Aklimatyzacja i Nasiennictwo 14(2):95–133
Kumar MS, Mawlong I, Nanjundan J, Aravind J, Singh D (2017) Seed color as an Index for assessing rapeseed meal quality. Bioscan 12(2):995–999
Larson SR, Young KA, Cook A, Blake TK, Raboy V (1998) Linkage mapping of two mutations that reduce phytic acid content of barley grain. Theor Appl Genet 97(1–2):141–146
Larson SR, Rutger JN, Young KA, Raboy V (2000) Isolation and genetic mapping of a non‐lethal rice (Oryza sativa L.) low phytic acid 1 mutation. Crop Sci 40(5):1397–405
Mawlong I, Sujith Kumar MS, Gurung B, Singh KH, Singh D (2017) A simple spectrophotometric method for estimating total glucosinolates in mustard de-oiled cake. Int J Food Prop 20(12):3274–3281
Mawlong I, Kumar MS, Kandpal BK, Premi OP, Gurung B, Singh D (2017) Meal and oil quality among genotypes of Indian mustard (Brassica juncea) varies under recommended dose of nitrogen fertilizer. Appl Ecol Environ Res 15(4):1427–1445
Mawson R, Heaney RK, Zdunczyk Z, Kozłowska H (1994) Rapeseed meal-glucosinolates and their antinutritional effects. Part 4. Goitrogenicity and internal organs abnormalities in animals. Die Nahrung 38(2):178–91
Naczk M, Amarowicz R, Sullivan A, Shahidi F (1998) Current research developments on polyphenolics of rapeseed/canola: a review. Food Chem 62(4):489–502
Pastuszewska B, Jabłecki G, Buraczewska L, Dakowski P, Taciak M, Matyjek R, Ochtabińska A (2003) The protein value of differently processed rapeseed solvent meal and cake assessed by in vitro methods and in tests with rats. Anim Feed Sci Technol 106(1–4):175–188
Hautfenne A (1987) Standard methods for the analysis of oils, fats and derivatives. Paquot C, editor. Oxford: Blackwell scientific publications
Peng J, Slominsiki BA, Guenter W, Campbell LD, Xiong YZ (2001) The anti-nutritional factors in Chinese double-low rapeseed meal. J Chinese Cereals Oils Assoc 16:6–10
Raboy V, Gerbasi PF, Young KA, Stoneberg SD, Pickett SG, Bauman AT, Murthy PP, Sheridan WF, Ertl DS (2000) Origin and seed phenotype of maize low phytic acid 1–1 and low phytic acid 2–1. Plant Physiol 124(1):355–368
Raboy V (2001) Seeds for a better future:‘low phytate’grains help to overcome malnutrition and reduce pollution. Trends Plant Sci 6(10):458–462
Sang JP, Salisbury PA (1988) Glucosinolate profiles of international rapeseed lines (Brassica napus and Brassica campestris). J Sci Food Agric 45(3):255–261
Sadeghi MA, Rao AA, Bhagya S (2006) Evaluation of mustard (Brassica juncea) protein isolate prepared by steam injection heating for reduction of antinutritional factors. LWT—Food Sci Technol 39(8):911–917
Selvam R (2002) Calcium oxalate stone disease: role of lipid peroxidation and antioxidants. Urol Res 30(1):35–47
Shamsuddin AM (2002) Anti-cancer function of phytic acid. Int J Food Sci Technol 37(7):769–782
Singh BK, Bala M, Rai PK (2014) Fatty acid composition and seed meal characteristics of Brassica and allied genera. Natl Acad Sci Lett 37(3):219–226
Velasco L, Becker HC (2000) Variability for seed glucosinolates in a germplasm collection of the genus Brassica. Genet Resour Crop Evol 47(3):231–238
Vreugdenhil D, Aarts MG, Koornneef M, Nelissen H, Ernst WH (2004) Natural variation and QTL analysis for cationic mineral content in seeds of Arabidopsis thaliana. Plant Cell Environ 27(7):828–839
Warwick SI, Gugel RK, McDonald T, Falk KC (2006) Genetic variation of Ethiopian mustard (Brassica carinata A. Braun) germplasm in western Canada. Genet. Resour. Crop Evol. 53(2):297–312
White PJ, Broadley MR (2005) Biofortifying crops with essential mineral elements. Trends Plant Sci 10(12):586–593
Wilcox JR, Premachandra GS, Young KA, Raboy V (2000) Isolation of high seed inorganic P, low-phytate soybean mutants. Crop Sci 40(6):1601–1605
Zrybko CL, Fukuda EK, Rosen RT (1997) Determination of glucosinolates in domestic and wild mustard by high-performance liquid chromatography with confirmation by electrospray mass spectrometry and photodiode-array detection. J Chromatogr A 767(1–2):43–52
Author Information
ICAR-Directorate of Rapeseed-Mustard Research, Sewar, India