Illigera Grandiflora W.W. Sm. & Jeffrey (Hernandiaceae): a potent source of antioxidants and in silico molecular docking analysis as vascular endothelial growth factor (VEGF) pathway regulator
*Article not assigned to an issue yet
Pathak Ashutosh, Das Rahul, Vijayan Deepu, Odyuo Nripemo, Baite David Lalsama, Mao Ashiho Asosii
Research Articles | Published: 21 February, 2025
First Page: 0
Last Page: 0
Views: 823
Keywords: Illigera grandiflora, LC-MS, Pharmacokinetics, pkCSM
Abstract
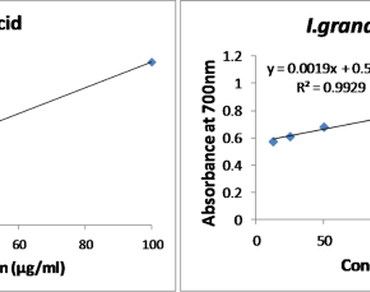
New insights into the drug discovery and development were based on the traditional knowledge. Tubers of Illigera grandiflora were used traditionally for the treatment of dropsy and traumatic injury in Traditional Chinese Medicine while ethnomedicinal knowledge present in Khiamniungan tribe, Nagaland, India reveals the role of I. grandiflora leaves in wound healing. In the present study, Illigera grandiflora W.W. Sm. & Jeffrey (Hernandiaceae) was evaluated for phytochemical compounds, antioxidant properties, detection of active compounds and probable mechanisms of action in wound healing. The methanolic leaf extract exhibited promising results in three antioxidants assays viz. DPPH, ABTS and Reducing Power Assay, compared to ascorbic acid as standard. (-)-Grandiflorimine/(-)-Grandifloramine, Methyl Vanillate, Ovigerine, Reticuline and Roemrefidine were identified through LC-MS. Reticuline (isoquinoline alkaloid) exhibited the most potent pharmacokinetic properties based on pkCSM models. The probable molecular mechanism of mode of action in present study was investigated through the potential of reticuline in inhibiting the HIF-1alpha degrading prolyl hydroxylase (PHDs). PHDs degrades the HIF-1 alpha under normoxia condition while under hypoxia PHDs are deactivated resulting into the activation of vascular endothelial growth factor (VEGF) pathway by HIF-1alpha. Reticuline exhibits the potential of binding PHDs thus which may trigger VEGF pathway under normoxia conditions leading to several important physiological processes such as vasodilation of blood vessels, increases permeability of blood vessel wall, and promotion of growth and proliferation of endothelial cells that lines blood vessels. Medicinal properties such as analgesic, CNS stimulant, Dopamine receptor blocking effect, and many more were already associated and reported for reticuline suggesting in multiple mode of molecular mechanisms.
References
Al Jaafreh AM (2024) Investigation of the phytochemical profiling and antioxidant, anti-diabetic, anti-inflammatory, and MDA-MB-231 cell line antiproliferative potentials of extracts from Ephedra Alata Decne. Sci Rep 14(1):18240
Berman HM, Westbrook J, Feng Z, Gilliland G, Bhat TN, Weissig H, Shindyalov IN, Bourne PE (2000) The Protein Data Bank. Nucleic Acids Res 28(1):235–242. https://doi.org/10.1093/nar/28.1.235
Bermejo A, Protais P, Blazouez MA, Rao KS, Zafra-Polo MC, Cortes D (1995) Dopaminergic isoquinoline alkaloids from roots of Xylopia papuana. Nat Prod Lett 6(1):57
Böhlke M, Guinaudeau H, Angerhofer CK, Wongpanich V, Soejarto DD, Farnsworth NR, Mora GA, Poveda LJ (1996) Costaricine, a new antiplasmodial bisbenzylisoquinoline alkaloid from Nectandra salicifolia trunk bark. J Nat Prod 59(6):576–580. https://doi.org/10.1021/np960195h
CADD (2019) https://cactus.nci.nih.gov
Chen IS, Chen JJ, Duh CY, Tsai IL, Chang CT (1997a) New aporphine alkaloids and cytotoxic constituents of Hernandia Nymphaeifolia. Planta Med 63(2):154–157. https://doi.org/10.1055/s-2006-957634
Chen JJ, Chang YL, Teng CM, Chen IS (2000) Anti-platelet aggregation alkaloids and lignans from Hernandia Nymphaeifolia. Planta Med 66(3):251–256. https://doi.org/10.1055/s-2000-8562
Chen JJ, Chang YL, Teng CM, Chen IS (2001) Vasorelaxing and antioxidant constituents from Hernandia Nymphaeifolia. Planta Med 67(7):593–598. https://doi.org/10.1055/s-2001-17348
Chen KS, Wu YC, Teng CM, Ko FN, Wu TS (1997b) Bioactive alkaloids from Illigera luzonensis. J Nat Prod 60(6):645–647. https://doi.org/10.1021/np9700735
Cheruthazhakkat S, Palamadathil Kozhicheri M, Erayur Mana A, Balachandran I (2024) Metabolite profiling of bark and secondary branches of Holarrhena pubescens Wall. Ex G. Don by chromatographic and tandem mass spectroscopic analyses. Vegetos, pp 1–16
Chimera (2016) https://www.cgl.ucsf.edu/chimera
Conserva LM, Pereira Cde A, Barbosa-Filho JM (2005) Alkaloids of the Hernandiaceae: occurrence and a compilation of their biological activities. Alkaloids Chem Biol 62:175–243. https://doi.org/10.1016/s1099-4831(05)62003-2
Dias KL, Da Silva Dias C, Barbosa-Filho JM, Almeida RN, De Azevedo Correia N, Medeiros IA (2004) Cardiovascular effects induced by reticuline in normotensive rats. Planta Med 70(4):328–333. https://doi.org/10.1055/s-2004-818944
Dimitrova P, Doncheva T, Kostova N, Uzunova I, Latinova N, Gerasimova V, Dat NT, Luyen NT (2024) Biological effect of alkaloid enriched fractions and reticuline from the Stephania Dielsiana Y. C. Wu on promyelocyte HL-60 cell line. Revista Brasileira de Farmacognosia (pre-print)
Freedman SJ, Sun ZY, Poy F, Kung AL, Livingston DM, Wagner G, Eck MJ (2002) Structural basis for recruitment of CBP/p300 by hypoxia-inducible factor-1 alpha. Proc Natl Acad Sci U S A 99(8):5367–5372. https://doi.org/10.1073/pnas.082117899
Gu JQ, Park EJ, Totura S, Riswan S, Fong HH, Pezzuto JM, Kinghorn AD (2002) Constituents of the twigs of Hernandia ovigera that inhibit the transformation of JB6 murine epidermal cells. J Nat Prod 65(7):1065–1068. https://doi.org/10.1021/np020042w
Huang LE, Pete EA, Schau M, Milligan J, Gu J (2002) Leu-574 of HIF-1alpha is essential for the Von Hippel-Lindau (VHL)-mediated degradation pathway. J Biol Chem 277(44):41750–41755. https://doi.org/10.1074/jbc.M207280200
Kageyama Y, Koshiji M, To KK, Tian YM, Ratcliffe PJ, Huang LE (2004) Leu-574 of human HIF-1alpha is a molecular determinant of prolyl hydroxylation. FASEB J 18(9):1028–1030. https://doi.org/10.1096/fj.03-1233fje
Kimura I, Chui LH, Fujitani K, Kikuchi T, Kimura M (1989) Inotropic effects of (+/-)-higenamine and its chemically related components, (+)-R-coclaurine and (+)-S-reticuline, contained in the traditional sino-japanese medicines bushi and shin-i in isolated guinea pig papillary muscle. Jpn J Pharmacol 50(1):75–78. https://doi.org/10.1254/jjp.50.75
Kumar CS, Narasu ML, Singh CR (2023) Pharmacokinetic analysis and structural optimization of inophyllamine-I to forecast as a possible drug candidate. Phytomedicine Plus 3(2):100422
Kumar H, Choi DK (2015) Hypoxia Inducible Factor Pathway and Physiological Adaptation: A Cell Survival Pathway? Mediators Inflamm. 2015:584758. https://doi.org/10.1155/2015/584758
Li X, Dong J, Gan D, Zhou D, Cai X, Cai L, Ding Z (2021) (-)-Grandiflorimine, a new dibenzopyrrocoline alkaloid with cholinesterase inhibitory activity from Illigera Grandiflora. Nat Prod Res 35(5):763–769. https://doi.org/10.1080/14786419.2019.1608542
Lin C, McGough R, Aswad B, Block JA, Terek R (2004) Hypoxia induces HIF-1alpha and VEGF expression in chondrosarcoma cells and chondrocytes. J Orthop Res 22(6):1175–1181. https://doi.org/10.1016/j.orthres.2004.03.002
Mfotie Njoya E, Munvera AM, Mkounga P, Nkengfack AE, McGaw LJ (2017) Phytochemical analysis with free radical scavenging, nitric oxide inhibition and antiproliferative activity of Sarcocephalus pobeguinii extracts. BMC Complement Altern Med 17(1):199. https://doi.org/10.1186/s12906-017-1712-5
Morais LC, Barbosa-Filho JM, Almeida RN (1998) Central depressant effects of reticuline extracted from Ocotea duckei in rats and mice. J Ethnopharmacol 62(1):57–61. https://doi.org/10.1016/s0378-8741(98)00044-0
Nakaoji K, Nayeshiro H, Tanahashi T (1997) Norreticuline and reticuline as possible new agents for hair growth acceleration. Biol Pharm Bull 20(5):586–588. https://doi.org/10.1248/bpb.20.586
National Center for Biotechnology Information. PubChem Database. Reticuline, National Center for Biotechnology Information, CID = 439653 https://pubchem.ncbi.nlm.nih.gov/compound/Reticuline
Nguyen TTU, Nguyen PT, Nguyen TT, Nguyen TPT, Dang CT, Nguyen HT (2024) Phytochemical profile, antioxidant and antibacterial activities of the ethanolic rice (Oryza sativa) leaf extract. Vegetos, pp 1–8
Okafor CE, Ijoma IK, Igboamalu CA, Ezebalu CE, Eze CF, Osita-Chikeze JC, Uzor CE, Ekwuekwe AL (2024) Secondary metabolites, spectra characterization, and antioxidant correlation analysis of the polar and nonpolar extracts of Bryophyllum pinnatum (Lam). Oken BioTechnologia 105(2):121–136
Pekkala M, Hieta R, Bergmann U, Kivirikko KI, Wierenga RK, Myllyharju J (2004) The peptide-substrate-binding domain of collagen prolyl 4-hydroxylases is a tetratricopeptide repeat domain with functional aromatic residues. J Biol Chem 279(50):52255–52261. https://doi.org/10.1074/jbc.M410007200
Pires DE, Blundell TL, Ascher DB (2015) pkCSM: Predicting Small-Molecule Pharmacokinetic and Toxicity properties using graph-based signatures. J Med Chem 58(9):4066–4072. https://doi.org/10.1021/acs.jmedchem.5b00104
Pushpa RRNS, Babulal KS, Kumaran R, Shoba G, Balakumaran MD (2023) In silico molecular interaction analysis and pharmacokinetic profiling of flavonoids from Catharanthus roseus (flower) against TXNIP protein. Trends Sci 20(1):6394–6394
Rahman M, Buragohain R, Das R, Islam MA, Hazarika NK, Pathak K, Barman P (2024) Comparative biochemical evaluation of the proximate content, antioxidant properties, and phytochemical constituents of ethnomedicinally important Docynia indica from Northeast India. Vegetos, pp 1–8
Shapovalov MV, Dunbrack RL Jr (2011) A smoothed backbone-dependent rotamer library for proteins derived from adaptive kernel density estimates and regressions. Structure 19(6):844–858. https://doi.org/10.1016/j.str.2011.03.019
Sylvie DD, Anatole PC, Cabral BP, Veronique PB (2014) Comparison of in vitro antioxidant properties of extracts from three plants used for medical purpose in Cameroon: Acalypha racemosa, Garcinia Lucida and Hymenocardia Lyrata. Asian Pac J Trop Biomed 4(Suppl 2):S625–S632
Trott O, Olson AJ (2010) AutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J Comput Chem 31(2):455–461. https://doi.org/10.1002/jcc.21334
Van Molle I, Thomann A, Buckley DL, So EC, Lang S, Crews CM, Ciulli A (2012) Dissecting fragment-based lead discovery at the Von Hippel-Lindau protein:hypoxia inducible factor 1α protein-protein interface. Chem Biol 19(10):1300–1312. https://doi.org/10.1016/j.chembiol.2012.08.015
Wang J, Wang W, Kollman PA, Case DA (2006) Automatic atom type and bond type perception in molecular mechanical calculations. J Mol Graph Model 25(2):247–260. https://doi.org/10.1016/j.jmgm.2005.12.005
Zhang Z, Li Y, Lin B, Schroeder M, Huang B (2011) Identification of cavities on protein surface using multiple computational approaches for drug binding site prediction. Bioinformatics 27(15):2083–2088. https://doi.org/10.1093/bioinformatics/btr331
Author Information
Forest Botany Division, Forest Research Institute, Dehradun, India