In silico structural analysis and ligand-binding predictions of a few developmental stage specific-proteins during in vitro morphogenesis in Vanilla
Research Articles | Published: 15 July, 2020
First Page: 570
Last Page: 579
Views: 3803
Keywords: Vanilla , In vitro morphogenesis, Proteins, Homology modeling, Molecular docking
Abstract
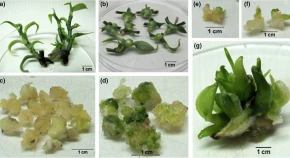
Vanilla (Vanilla planifolia Jacks. ex Andrews) is an orchid. It is adored for vanillin, a unique flavouring principle, used in numerous products. The production of Vanilla is dependent on in vitro mass propagation. Since it is a recalcitrant plant, its callus-based regeneration is often enigmatic. The present study reports a few development-stage specific proteins, their structural analysis and ligand-binding predictions, which are essential for the undifferentiated callus of Vanilla during the transition to the differentiated state. Of these proteins, the up regulation of Light-independent protochlorophyllide reductase in the dark grown-callus and retaining the same when the callus was placed under light after subculture to regeneration medium possibly indicated that it helped the callus to acquire its autotrophic stature that is vital for its self-sustenance in the differentiated state. Additionally, the up regulation of Phloem protein 2 in the callus and regenerating callus is an important finding since translocation of solutes is of utmost importance for the formation of the conductive elements, which are necessary for the development of the organized structures for the undifferentiated tissue to convert into the differentiated state.
References
- Armstrong G (1998) Greening in the dark: light independent chlorophyll biosynthesis from anoxygenic photosynthetic bacteria to gymnosperms. J Photochem Photobiol B Biol 43:87–100
- Balen B, Rasol MK, Zadro I, Rudolf SV (2004) Esterase activity and isoenzymes in relation to morphogenesis in Mammillaria gracillis Pfeiff. tissue culture. Acta Bot Croat 63:83–91
- Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
- Carpentier SC, Witters E, Laukens K, Deckers P, Swennen R, Panis B (2005) Preparation of protein extracts from recalcitrant plant tissues: an evaluation of different methods for two-dimensional gel electrophoresis analysis. Proteomics 5:2497–2507
- Caverzan A, Passaia G, Rosa SB, Ribeiro CW, Lazzarotto F, Margis-Pinheiro M (2012) Plant responses to stresses: role of ascorbate peroxidase in the antioxidant protection. Genet Mol Biol 35:1011–1019
- Cherian MG, Kang YJ (2006) Metallothionein and liver cell regeneration. Exptl Biol Med 231:138–144
- Chevallet M, Luche S, Rabilloud T (2006) Silver staining of proteins in polyacrylamide gels. Nat Protoc 14:1852–1858
- Damerval C, de Vienne D, Zivy M, Thiellement H (1986) Technical improvements in two-dimensional electrophoresis increase the level of genetic variation detected in wheat-seedling proteins. Electrophoresis 7:52–54
- Dinant S, Clark AM, Zhu Y, Vilaine F, Palauqui JC, Kusiak C, Thompson GA (2003) Diversity of the super family of phloem lectins (phloem protein 2) in angiosperms. Plant Physiol 131:114–128
- Gallage NJ, Hansen EH, Kannangara R, Olsen CE, Mo Motawia MS, Jorgensen K, Holme I, Hebelstrup K, Grisoni M, Moller BL (2014) Vanillin formation from ferulic acid in Vanilla planifolia is catalysed by a single enzyme. Nat Commun 5:4037
- Gharahdaghi F, Weinberg CR, Meagher DA, Imai BS, Mische SM (1999) Mass spectrometric identification of proteins from silver-stained polyacrylamide gel: a method for the removal of silver ions to enhance sensitivity. Electrophoresis 20:601–605
- Grennan AK (2011) Metallothioneins, a diverse protein family. Plant Physiol 155:1750–1751
- Hochholdinger F, Sauer M, Dembinsky D, Hoecker N, Muthreich N, Saleem M, Liu Y (2006) Proteomic dissection of plant development. Proteomics 6:4076–4083
- Ikeuchi M, Ogawa Y, Iwase A, Sugimoto K (2016) Plant regeneration: cellular origins and molecular mechanisms. Development 143:1442–1451
- Machado MFPS, Prioli AJ, Mangolin CA (1993) Malate dehydrogenase (MDH; EC 1.1.1.37) isozymes in tissues and callus cultures of cereusperuvianus (Cactaceae). Biochem Genet 31:167–172
- Palama TL, Menard P, Fock I, Choi YH, Bourdon E, Govinden-Soulange J, Bahut M, Payet B, Verpoorte R, Kodja H (2010) Shoot differentiation from protocorm callus cultures of Vanilla planifolia (Orchidaceae): proteomic and metabolic responses at early stage. BMC Plant Biol 10:82
- Schmitz-Linneweber C, Small I (2008) Pentatricopeptide repeat proteins: a socket set for organelle gene expression. Trend Plant Sci 13:663–670
- Sultana M, Gangopadhyay G (2014) Looking for isoforms of enzymes related to in vitro morphogenesis in Nicotiana tabacum L. Int Res J Biol Sci 3:11–16
- Tan BC, Chin CF, Alderson P (2011) Optimisation of plantlet regeneration from leaf and nodal derived callus of Vanilla planifolia Andrews. Plant Cell Tiss Organ Cult 105:457–463
- Tan BC, Chin CF, Liddell S, Alderson P (2013) Proteomic analysis of callus development in Vanilla planifolia Andrews. Plant Mol Biol Rep 31:1220–1229
- Wetter L, Dyck J (1983) Isozyme analysis of cultured cells and somatic hybrids. In: Evans DA, Sharp WR, Ammirato PV, Yamada Y (eds) Handbook of plant cell culture, 1. Macmillam Publishing Co., New York, pp 607–627
- Yang J, Roy A, Zhang Y (2013a) Protein-ligand binding site recognition using complementary binding-specific substructure comparison and sequence profile alignment. Bioinformatics 29:2588–2595
- Yang J, Roy A, Zhang Y (2013b) BioLiP: a semi-manually curated database for biologically relevant ligand-protein interactions. Nucleic Acids Res 41:D1096–D1103
- Zhang Y (2008) I-TASSER server for protein 3D structure prediction. BMC Bioinf. https://doi.org/10.1186/1471-2105-9-40
Author Information
Division of Plant Biology, Bose Institute, Kolkata, India