In vitro and silico assessment of berbericinine, a promising drug candidate against neurodegeneration and neuroinflammation
*Article not assigned to an issue yet
Research Articles | Published: 14 October, 2024
First Page: 0
Last Page: 0
Views: 1619
Keywords: Aβ, Alzheimer’s disease, Berbericinine, Docking, Receptor-ligand, Interaction, In vitro, In silico
Abstract
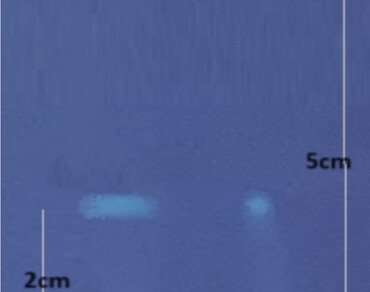
To perform in vitro and silico assessments of berbericinine, and to assess its protective effect against neurodegeneration and neuroinflammation. Isolation and characterization of berbericinine from Tinospora cordifolia leaves followed by TLC, and HPLC studies. In vitro anti-inflammatory activity, free radical scavenging action, and choline esterase inhibitory studies were performed followed by in silico toxicity studies and docking studies. The TLC and HPLC studies compared to the standard confirmed the purity of isolated berbericinine from Tinospora cordifolia leaves. Findings of TLC with HPLC confirmed the presence of said bioactive compound with Rf of 0.4 and Rt of 20.82 min. DPPH and FRAP assays confirmed the potent antioxidant nature of berbericinine (IC50 of 29.04 µg/mL and TEAC of 56.35 µMol). Further, the in vitro anti-inflammatory study revealed that extract and isolated berbericinine inhibited albumin denaturation and showed potent anti-proteinase, and anti-lipoxygenase activities. The findings indicated that isolated berbericinine showed higher anti-inflammatory activity than the extract. The in silico approach aided the identification of the possible targets of action of berbericinine in the brain. Results of docking studies suggest that berbericinine showed a high docking score of − 7.6 kcal/mol with MAP kinase (1CM8), − 7.8 kcal/mol with β-secretase (2OHM), − 7.2 kcal/mol with NF-KB (6M2D), and − 7.4 kcal/mol with EGFR (4D90) and resulted in robust binding affinities with key molecular targets. Furthermore, berbericinine revealed a potent in vitro acetylcholine esterase inhibitory effect in a dose-dependent manner. The research findings indicate that isolated berbericinine shows promising potential in interacting with and influencing critical pathways, particularly relevant in the context of neurodegenerative disorders like Alzheimer s disease.
References
Bajpai V, Singh A, Chandra P, Negi MPS, Kumar N, Kumar B (2016) Analysis of phytochemical variations in dioecious Tinospora cordifolia stems using HPLC/QTOF MS/MS and UPLC/QqQ-MS/MS. PCA 27(2):92–99. https://doi.org/10.1002/pca.2601
Biovia D, Berman H, Westbrook J, Feng Z, Gilliland G, Bhat T, Richmond TJOCP (2016) Dassault systèmes BIOVIA, discovery studio visualizer, v. 17.2. San Diego: Dassault Systèmes 10(2000):0021–9991
Bondy SC (2021) Metal toxicity and neuroinflammation. Curr Opin Toxicol 26:8–13. https://doi.org/10.1016/j.cotox.2021.03.008
Chen Y, Yu Y (2023) Tau and neuroinflammation in AD disease: interplay mechanisms and clinical translation. J Neuroinflammation 20:165
Chiarini A, Armato U, Hu P, Prà ID (2020) Danger-sensing/patten recognition receptors and neuroinflammation in AD disease. Int J Mol Sci 21:9036. https://doi.org/10.3390/ijms21239036
Corrêa SA, Eales KL (2012) The role of p38 MAPK and its substrates in neuronal plasticity and neurodegenerative disease. J Signal Transduct. https://doi.org/10.1155/2012/649079
Ellman GL, Courtney KD, Andres V, Featherstone RM (1961) A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol 7(2):88–95. https://doi.org/10.1016/0006-2952(61)90145-9
Ercal N, Gurer-Orhan H, Aykin-Burns N (2001) Toxic metals and oxidative stress part I: mechanisms involved in metal-induced oxidative damage. Curr Top Med Chem 1(6):529–539. https://doi.org/10.2174/1568026013394831
Eshwarappa RS, Ramachandra YL, Subaramaihha SR, Subbaiah SG, Austin RS, Dhananjaya BL (2016) Anti-lipoxygenase activity of leaf gall extracts of terminalia chebula (Gaertn) Retz. (Combretaceae). Pharmacognosy Res. 8(1):78–82
Forli S, Huey R, Pique ME, Sanner MF, Goodsell DS, Olson AJ (2016) Computational protein-ligand docking and virtual drug screening with the AutoDock suite. Nat Protoc 11(5):905–919. https://doi.org/10.1038/nprot.2016.051
Ge ZC, Zhou JM (2004) Determination of berberine, berbericinine and jatrorrhizine in coptis and its preparations by micellar thin layer chromatographic scanning method. eCJac. 32(1):99–101
Ghosh AK, Brindisi M, Tang J (2012) Developing β-secretase inhibitors for the treatment of AD disease. J Neurochem 120(1):71–83
Hsieh TJ, Chia YC, Wu YC, Chen CY (2004) Chemical constituents from the stems of Mahonia japonica. J Chinese Chem Society 51(2):443–446. https://doi.org/10.1002/jccs.200400068
Huang YH, Rose PW, Hsu CN (2015) Citing a data repository: a case study of the protein data bank. PLoS ONE 10(8):e0136631. https://doi.org/10.1371/journal.pone.0136631
Huat TJ, Camats-Perna J, Newcombe EA, Valmas N, Kitazawa M, Medeiros R (2019) Metal toxicity links to alzheimer’s disease and neuroinflammation. J Mol Biol 431(9):1843–1868. https://doi.org/10.1016/j.jmb.2019.01.018
Ali H, Dixit S (2013) Extraction optimization of Tinospora cordifolia and assessment of the anticancer activity of its alkaloid berbericinine. Sci World J. https://doi.org/10.1155/2013/376216
Inestrosa NC, Dinamarca MC, Alvarez A (2008) Amyloid–cholinesterase interactions implications for alzheimer’s disease. FEBS J 275:625–632. https://doi.org/10.1111/j.1742-4658.2007.06238.x
Joanna KC, Daria S, Paulina M, Dominik K, Anna GM, Bartosz K, Judyta CP (2018) In vitro screening for acetylcholinesterase and butyrylcholinesterase inhibition and antimicrobial activity of chia seeds (Salvia hispanica). Electron J Biotechnol 37:1–10. https://doi.org/10.1016/j.ejbt.2018.10.002
Johnson VJ, Sharma RP (2003) Aluminum disrupts the pro-inflammatory cytokine/neurotrophin balance in primary brain rotation-mediated aggregate cultures: possible role in neurodegeneration. NeuroToxicol 24(2):261–268
Jung J, Choi JS, Jeong CS (2014) Inhibitory activities of palmatine from coptis chinensis against helicobactor pylori and gastric damage. Toxicol Res 30(1):45–48. https://doi.org/10.5487/TR.2014.30.1.045
Kumar P, Srivastava V, Chaturvedi R, Sundar D, Bisaria VS (2017) Elicitor enhanced production of protoberberine alkaloids from in vitro cell suspension cultures of Tinospora cordifolia (Willd.) Miers. Ex. Hook. F & Thoms. PCTOC. 130(2):417–426. https://doi.org/10.1007/s11240-017-1237-0
Li J, Wu Y, Dong S, Yu Y, Wu Y, Xiang B, Li Q (2023) Research progress on neuroprotective effects of isoquinoline alkaloids. MOL 28(12):4797. https://doi.org/10.3390/molecules28124797
Ma B, Zhu L, Zang X, Chen Y, Li D, Wang Y (2013) Coptis chinensis inflorescence and its main alkaloids protect against ultraviolet-B-induced oxidative damage. J Funct Foods 5(4):1665–1672. https://doi.org/10.1016/j.jff.2013.07.010
Mensor LL, Menezes FS, Leitão GG, Reis AS, dos Santos TC, Coube CS, Leitão SG (2001) Screening of Brazilian plant extracts for antioxidant activity by the use of DPPH free radical method. Phytother Res 15(2):127–130. https://doi.org/10.1002/ptr.687
Miao J, Ma H, Yang Y, Liao Y, Lin C, Zheng J, Yu M, Lan J (2023) Microglia in Alzheimer’s disease: pathogenesis, mechanisms, and therapeutic potentials. Front Aging Neurosci 15:1201982. https://doi.org/10.3389/fnagi.2023.1201982
Mukherjee PK, Kumar V, Mal M, Houghton PJ (2007) Acetylcholinesterase Inhibitors from Plants. PYTOEY 14(4):289–300. https://doi.org/10.1016/j.phymed.2007.02.002
Mukund V, Behera SK, Alam A, Nagaraju GP (2019) Molecular docking analysis of nuclear factor-κB and genistein interaction in the context of breast cancer. Bioinformation 15(1):11–17
Murray CW, Callaghan O, Chessari G, Cleasby A, Congreve M, Frederickson M (2007) Application of fragment screening by X-ray crystallography to β-Secretase. J Med Chem 50(6):1116–1123. https://doi.org/10.1021/jm0611962
Norgan AP, Coffman PK, Kocher JPA (2011) Multilevel parallelization of AutoDock 4.2. J Cheminform 3:12. https://doi.org/10.1186/1758-2946-3-12
Purwaningsih I, Maksum IP, Sumiarsa D, Sriwidodo S (2024) Isolation and quantification of Palmatine from Fibraurea tinctoria Lour. in vitro antioxidant activity and in silico antidiabetic activity evaluation. Drug Des Devel Ther 18:3443–3459. https://doi.org/10.2147/DDDT.S454091
Qu Ws, Tian Ds, Guo Zb et al (2012) Inhibition of EGFR/MAPK signaling reduces microglial inflammatory response and the associated secondary damage in rats after spinal cord injury. J Neuroinflammation 9:178. https://doi.org/10.1186/1742-2094-9-178
Rajurkar NS, Hande SM (2011) Estimation of phytochemical content and antioxidant activity of some selected traditional Indian medicinal plants. Indian J Pharm Sci 73(2):146–151
Ren Y, Long S, Cao S (2021) Molecular docking and virtual screening of an influenza virus inhibitor that disrupts protein-protein interactions. Viruses 13(11):2229. https://doi.org/10.3390/v13112229
Rockenstein E, Mante M, Alford M, Adame A, Crews L, Hashimoto M, Esposito L, Mucke L, Masliah E (2005) High beta-secretase activity elicits neurodegeneration in transgenic mice despite reductions in amyloid-beta levels: implications for the treatment of Alzheimer disease. J Biol Chem 280(38):32957–32967. https://doi.org/10.1074/jbc.M507016200
Romano R, Bucci C (2020) Role of EGFR in the nervous system. Cells 9(8):1887. https://doi.org/10.3390/cells9081887
Sabrina Manel KS, Lekhmici A, Abderrahmane B (2020) Anti-Inflammatory potential evaluation (In-Vitro and In-Vivo) of Arthrophytum scoparium Aerial Part. JDDT 10(5):213–218
Sadigh-Eteghad S, Sabermarouf B, Majdi A, Talebi M, Farhoudi M, Mahmoudi J (2015) Amyloid-beta: a crucial factor in Alzheimer’s disease. Med Princ Pract 24(1):1–10. https://doi.org/10.1159/000369101
Sakat S, Juvekar AR, Gambhire MN (2010) In vitro antioxidant and anti-inflammatory activity of methanol extract of Oxalis corniculata Linn. Int J Pharm Pharm Sci 2:146–155
Saraswat J, Singh P, Patel R (2021) A computational approach for the screening of potential antiviral compounds against SARS-CoV-2 protease: Ionic liquid vs herbal and natural compounds. J Mol Liq 326:115298. https://doi.org/10.1016/j.molliq.2021.115298
Scheltens P, De Strooper B, Kivipelto M, Holstege H, Chételat G, Teunissen CE, Cummings J, van der Flier WM (2021) AD disease. Lancet 397:1577–1590. https://doi.org/10.1016/S0140-6736(20)32205-4
Schürpf T, Chen Q, Liu JH, Wang R, Springer TA, Wang J-H (2012) The RGD finger of Del-1 is a unique structural feature critical for integrin binding. FASEB J 26(8):3412–3420. https://doi.org/10.1096/fj.11-202036
Singh S, Singh TG (2020) Role of nuclear factor kappa B (NF-κB) signalling in neurodegenerative diseases: an mechanistic approach. Curr Neuropharmacol 18(10):918–935. https://doi.org/10.2174/1570159X18666200207120949
Son SH, Lee NR, Gee MS, Song CW, Lee SJ, Lee SK (2023) Chemical knockdown of phosphorylated p38 mitogen-activated protein kinase (MAPK) as a novel approach for the treatment of alzheimer′s disease. ACS Cent Sci 3:417–426. https://doi.org/10.1021/acscentsci.2c01369
Tang Y, Li S, Li S, Yang X, Qin Y, Liu C, Zhang Y (2017) Screening and isolating potential α-glucosidase inhibitors from Rhizoma Coptidis by ultrafiltration LC-PDA-ESI/MS combined with high-speed countercurrent chromatography and reverse-phase medium-pressure liquid chromatography. Med Chem Res. https://doi.org/10.1007/s00044-017-2031-6
Tavassoly O, Sato T, Tavassoly I (2020) Inhibition of brain EGFR activation: a novel target in neurodegenerative diseases and brain injuries. Mol Pharmacol 120:119909. https://doi.org/10.1124/mol.120.119909
Thawkar BS, Kaur G (2019) Inhibitors of NF-κB and P2X7/NLRP3/Caspase 1 pathway in microglia: novel therapeutic opportunities in neuroinflammation induced early-stage AD disease. J Neuroimmun 326:62–74. https://doi.org/10.1016/j.jneuroim.2018.11.010
Ting EY, Yang AC, Tsai SJ (2020) Role of interleukin-6 in depressive disorder. Int J Mol Sci 21(6):2194. https://doi.org/10.3390/ijms21062194
Walczak-Nowicka ŁJ, Herbet M (2021) Acetylcholinesterase inhibitors in the treatment of neurodegenerative diseases and the role of acetylcholinesterase in their pathogenesis. Int J Mol Sci 22:9290. https://doi.org/10.3390/ijms22179290
Yan B, Wang D, Dong S, Cheng Z, Na L, Sang M, Yang H, Yang Z, Zhang S, Yan Z (2017) Palmatine inhibits TRIF-dependent NF-κB pathway against inflammation induced by LPS in goat endometrial epithelial cells. Int Immunopharmacol 45:194–200. https://doi.org/10.1016/j.intimp.2017.02.004
Yang SB, Kim EH, Kim SH, Kim YH, Weontae L, Jang JT, Sabina YA, Yeasmin J, Byung C, Hyun YJ (2018) Electrospinning fabrication of poly(vinyl alcohol)/Coptis chinensis extract nanofibers for antimicrobial exploits. Nanomaterials 8(9):734. https://doi.org/10.3390/nano8090734
Zatta P, Drago D, Bolognin S, Sensi SL (2009) ADdisease, metal ions and metal homeostatic therapy. TIPS 30(7):346–355
Author Information
Department of Pharmacology, Krupanidhi College of Pharmacy, Bengaluru, India