In vitro plant regeneration of some recalcitrant indica rice (Oryza sativa L.) varieties
Research Articles | Published: 03 February, 2021
First Page: 102
Last Page: 106
Views: 4227
Keywords: Embryogenic callus, Oryza sativa , Regeneration, Tissue culture
Abstract
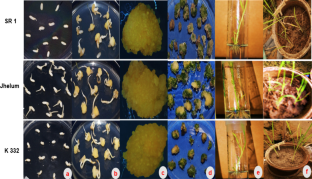
Rice is the staple diet for the greater part of the world’s population and there is a regular expanding need of rice production. The plant development and productivity are decreased by numerous factors. Utilizing biotechnological instruments is the most useful alternative to create rice varieties with incredible grain quality, improved productivity and protection from different stresses. Nonetheless, the deficiency of a simple, speedy and competent protocol for embryogenic callus induction and plant recovery is a significant draw back and there is substantial genotype-dependence. The current study explores the impact of plant growth regulators on the callus induction, proliferation and recovery from developed seeds in three rice varieties—SR 1, Jehlum and K 332. Greatest callus induction (82.33 ± 2.51% in SR1, 99.66 ± 0.57% in Jehlum and 93.33 ± 2.88% in K332) was seen in type 2 media containing 3% maltose, 500 mg/l casein hydrolysate, 500 mg/l l-proline, 5 mg/l l-glutamine, 40 mg/l cysteine, 5 mg/l l-asparagine, 100 mg/l ascorbic acid, 4 mg/l AgNO3, 600 mg/l MgCl2 enhanced with 4 g/l gelrite, 0.2 mg/l BAP and 2.5 mg/l 2, 4-D. Most noteworthy recurrence of shoot recovery (75.0 ± 3.0% in SR1, 86.66 ± 2.88% in Jehlum and 88.33 ± 2.88% in K332) was seen in the MS B5 medium containing 3 g/l phytagel enhanced with 4% sucrose, 2.5 mg/l BAP, 1 mg/l zeatin, 0.2 mg/l NAA and 0.5 mg/l TDZ. The current protocol gives a quick callus induction and recovery system, which could be utilized in effective genetic transformation and for creating genetically altered plants.
References
- Bajaj S, Mohanty A (2005) Recent advances in rice biotechnology-towards genetically superior transgenic rice. Plant Biotechnol J 3:275–307
- Bhojwani SS, Razdan MK (1996) Plant tissue culture, theory and practices. A revised edition. Elsevier Publishers, Amsterdam, pp 50–51
- Chevreau E, Mourgues F, Neveu M, Chevalier M (1997) Effect of gelling agents and antibiotics on adventitious bud regeneration from in vitro leaves of pear. Vitro Cell Dev Biol Plant 33:173–179
- Dey M, Bakshi S, Galiba G, Sahoo L, Pand SK (2012) Development of a genotype independent and transformation amenable regeneration system from shoot apex in rice (Oryza sativa spp. Indica) using TDZ. 3 Biotech 2(3):233–240
- Gamborg OL, Miller RA, Ojima K (1968) Nutrient requirements of suspension cultures of soybean root cells. Exp Cell Res 50:151–158
- Geng PP, La HG, Wang HQ, Stevens EJC (2008) Effect of sorbitol concentration on regeneration of embryogenic calli in upland rice varieties (Oryza sativa L.). Plant Cell Tiss Org Cult 92:303–313
- Joyia FA, Khan MS (2012) Reproducible and expedient rice regeneration system using in vitro grown plants. Afr J Biotechnol 11:138–144
- Karthikeyan A, Pandian STK, Ramesh M (2009) High frequency plant regeneration from embryogenic callus of a popular indica rice (Oryza sativa L.). Physiol Mol Biol Plants 15(4):371–375
- Khalequzzaman M, Haq N, Hoque M, Aditya T (2005) Regeneration efficiency and genotypic effect of 15 indica type Bangladeshi rice (Oryza sativa L.) Landraces. Plant Tissue Cult Biotechnol 15:33–42
- Khush GS (2005) What it will take to feed 5.0 billion rice consumers in 2030. Plant Mol Biol 59:1–6
- Kumar V, Shriram V, Kishor PBK, Jawali N, Shitole MG (2010) Enhanced proline accumulation and salt stress tolerance of transgenic Indica rice by over-expressing P5CSF129A gene. Plant Biotechnol Rep 4:37–48
- Lin YJ, Zhang QF (2005) Optimizing the tissue culture conditions for high efficiency transformation of Indica rice. Plant Cell Rep 23:540–547
- Meneses A, Flores D, Munoz M, Arrieta G, Espinoza AM (2005) Effect of 2,4-d, hydric stress and light on indica rice (Oryza sativa) somatic embryogenesis. Rev Biol Trop 53:361–368
- Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassay with tobacco tissue culture. Physiol Plant 15:473–497
- Nakagarwara S, Goto T, Nara M, Ozawa Y, Hotta K, Arata Y (1998) Spectroscopic characterization and the pH dependence of bacterial activity of the aqueous chlorine solution. Anim Sci 14:691–698
- Parvin VJ, Dudhare MS, Saluja T, Sarawgi AK, Saxena R, Girish C (2011) Assessment of critical factors influencing callus induction, in vitro regeneration and selection of bombarded indica rice genotypes. J Agric Biotechnol Sustain Dev 3(3):44–59
- Patpanukul T, Bunnag S, Theerakulpisut P, Kosittrakul M (2004) Transformation of indica rice (Oryza sativa L.) cv. RD6 mediated by Agrobacterium tumefaciens. Songklanakarin J Sci Technol 26:1
- Pazuki A, Sohani MM (2013) Phenotypic evaluation of scutellum-derived calluses in ‘Indica’ rice cultivars. Acta Agric Slovenica 101(2):239–247
- Pons MJ, Marfa V, Mele E, Massanger J (2000) Regeneration and genetic transformation of Spanish rice cultivars using mature embryo. Euphytica 114:117–122
- Rashid H, Saleem M, Chauhry Z, Gilani ST, Qureshi AS (2004) Studies on developing a high regeneration from seed derived calli of rice (Oryza sativa L.) cv. Super Basmati. Pak J Biol Sci 7(2):273–276
- Santarem ER, Pelissier B, Finer JJ (1997) Effect of explant orientation, pH, solidifying agent and wounding on initiation of soybean somatic embryos. Vitro Cell Dev Biol Plant 33:13–19
- Shahsavari E (2010) Evaluation and optimizations of media on the tissue culture system of upland rice. Int J Agric Biol 12:537–540
- Sharma C, Kaur M, Kaur A, Gosal SS (2012) In vitro plant regeneration studies in three indica rice varieties. Int J Agric Environ Biotechnol 5(4):309–313
- Wunn J, Kloti A, Burkhardt PK, Ghosh Biswas CG, Launis K, Iglesias VA, Potrykus I (1996) Transgenic Indica rice breeding line IR58 expressing a synthetic cryIA (b) gene from Bacillus thuringiensis provide effective insect pest control. Biol Technol 14:171–176
- Zaidi MA, Narayanan M, Sardana R, Taga I, Postel S, Johns R, McNulty M, Mottiar Y, Mao J, Loit E, Altosaar I (2006) Optimizing tissue culture media for efficient transformation of different indica rice genotypes. Agron Res 4:563–575
- Zhang Y, Chautler SE, Gupta S, Zhao Y, Hannah LC, Meyer C, Weston J, Wu MX, Preiss J, Okita TW (1996) Molecular approaches to enhance rice production through manipulation of starch metabolism during seed development. Rice genetics III, Proc. third international rice genetics symposium. Khush GS (ed.). IRRI, Manila, Philippines, pp 809–813
- Zhao LN, Zhou HJ, Lu LX, Liu L, Li XH, Lin YJ, Yu SB (2009) Identification of quantitative trait loci controlling rice mature seed culturability using chromosomal segment substitution lines. Plant Cell Rep 28:247–256
Author Information
Department of Botany, Sri Pratap College, Srinagar, India