Phytochemical characterization, antioxidant properties, and in-vitro antibacterial activity of three Piper betle cultivars
*Article not assigned to an issue yet
Research Articles | Published: 20 May, 2025
First Page: 0
Last Page: 0
Views: 1234
Keywords: Betel leaf, Antibacterial, Antioxidant, Chavicol, Eugenol
Abstract
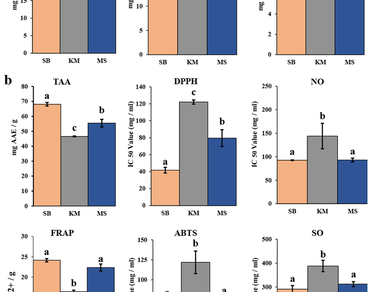
Piper betle L., an important cash crop in India, has a rich array of phytocompounds with promising bioactive properties beneficial for therapeutic development. Due to the emergence of rising multidrug resistance in bacteria, there is an urgent need for innovative therapies and novel antimicrobials. Considering this, the current study focuses on a comparative investigation of three P. betle cultivars: Simurali Bangla (SB), Kakdwip Meetha (KM), and Medinipore Sanchi (MS), aiming to explore their phytochemical content, antioxidant activities, and antibacterial properties using leaf methanolic extract. Among the cultivars, SB showed the highest total flavonoid (TFC; 19.30 ± 0.14 mg QE/g extract) and total tannin (TTC; 10.93 ± 0.25 mg GAE/g extract), while the total phenols (TPC; mg GAE/g extract) was highest in MS (30.40 ± 0.28) followed by SB (29.90 ± 0.14). Furthermore, six different antioxidant assays revealed the maximum efficacy in SB. This cultivar exhibited substantial antibacterial activity against Gram-negative and Gram-positive multidrug-resistant (MDR) bacterial strains. A correlation and multivariate analysis indicated positive correlations among polyphenolic content, antioxidant potential, and antibacterial activity grouped SB and MS, separating KM. The Gas Chromatography-Mass Spectrometry (GC-MS) analysis identified eugenol and chavicol derivatives as the major compounds. Quantitation of eugenol and chavicol by High-Pressure Liquid Chromatography (HPLC) revealed higher contents in SB and MS than KM. The results provide valuable insights into the potential health benefits of P. betle, especially the SB, in identifying natural antioxidants and plant-derived antimicrobials.
References
Abdelli M, Moghrani H, Aboun A, Maachi R (2016) Algerian Mentha pulegium L. leaves essential oil: chemical composition, antimicrobial, insecticidal and antioxidant activities. Ind Crops Prod 94:197–205
Agarwal T, Singh R, Shukla AD, Waris I, Gujrati A (2012) Comparative analysis of antibacterial activity of four Piper betle varieties. Adv Appl Sci Res 3(2):698–705
Bauer AW, Kirby WM, Sherris JC, Turck M (1966) Antibiotic susceptibility testing by a standardized single disk method. Am J Clin Pathol 45:493–496
Bhuvaneswari S, Sripriya N, Deepa S, Prakash NKU (2014) Studies on antioxidant activities of six cultivars of Piper betle L. Int J Pharm Sci 6:270–273
Borges CV, Junior SS, Lima FSP, Simons GPP (2018) Agronomic factors influencing brassica productivity and phytochemical quality. In: El-Esawi MA (ed) Brassica Germplasm - characterization, breeding and utilization, 1st edn. IntechOpen, Austria, pp 57–74. https://doi.org/10.5772/intechopen.74732
Bueno-Costa FM, Zambiazi RC, Bohmer BW, Chaves FC, da Silva WP, Zanusso JT, Dutra I (2016) Antibacterial and antioxidant activity of honeys from the state of Rio Grande do Sul, Brazil. LWT-Food Sci Technol 65:333–340
Catherine AA, Deepika H, Negi PS (2012) Antibacterial activity of Eugenol and peppermint oil in model food systems. J Essent Oil Res 24(5):481–486
Cebi N, Yilmaz MT, Sagdic O (2017) A rapid ATR-FTIR spectroscopic method for detection of sibutramine adulteration in tea and coffee based on hierarchical cluster and principal component analyses. Food Chem 229:517–526
Cosme P, Rodríguez AB, Espino J, Garrido M (2020) Plant phenolics: bioavailability as a key determinant of their potential health-promoting applications. Antioxidants 9(12):1263. https://doi.org/10.3390/antiox9121263
Elufioye TO, Olusola DM, Oyedeji AO (2019) Correlation of total phenolic, flavonoid and tannin content of Bryophyllum pinnatum (Lam.) (crassulaceae) extract with the antioxidant and anticholinesterase activities. Pharmacogn J 11(5):1003–1009. https://doi.org/10.5530/pj.2019.11.158
Fair RJ, Tor Y (2014) Antibiotics and bacterial resistance in the 21st century. Perspect Med Chem 6(6):S14459. https://doi.org/10.4137/pmc.s14459
Guha P (2006) Betel leaf: the neglected green gold of India. J Hum Ecol 19:87–93
Gupta RK, Guha P, Srivastav PP (2023) Phytochemical and biological studies of betel leaf (Piper Betle L.): review on paradigm and its potential benefits in human health. Acta Ecol Sin 43:721–732. https://doi.org/10.1016/j.chnaes.2022.09.006
Harini SS, Sougandhi PR, Tenkayala DSR, Gopinath KR (2018) Antioxidant activity (phenol and flavonoid content) of three different cultivars of Piper betle L. (Piperaceae). J Drug Deliv 8:143–148. https://doi.org/10.22270/jddt.v8i5-s.1978
Islam MA, Ryu KY, Khan N, Song OY, Jeong JY, Son JH, Jamila N, Kim KS (2020) Determination of the volatile compounds in five varieties of Piper betle L. from Bangladesh using simultaneous distillation extraction and gas chromatography/mass spectrometry (SDE-GC/MS). Anal Lett 53:2413–2430. https://doi.org/10.1080/00032719.2020.1744160
Karak S, Das S, Biswas M, Choudhury A, Dutta M, Chaudhury K, De B (2019) Phytochemical composition, β-glucuronidase Inhibition, and antioxidant properties of two fractions of Piper betle leaf aqueous extract. J Food Biochem 43:1–12. https://doi.org/10.1111/jfbc.13048
Khan AA, Bhatnagar SP, Sinha BN, Lal UR (2013) Pharmacognostic specifications of eight cultivars of Piper betle from Eastern region of India. Pharmacogn J 5:176–183. https://doi.org/10.1016/j.phcgj.2013.07.002
Klančnik A, Piskernik S, Jeršek B, Možina SS (2010) Evaluation of diffusion and Dilution methods to determine the antibacterial activity of plant extracts. J Microbiol Methods 81:121–126. https://doi.org/10.1016/j.mimet.2010.02.004
Kora AJ, Arunachalam J (2010) Assessment of antibacterial activity of silver nanoparticles on Pseudomonas aeruginosa and its mechanism of action. World J Microbiol 27:1209–1216. https://doi.org/10.1007/s11274-010-0569-2
Lin J, Gao X, Cui Y, Sun W, Shen Y, Shi Q, Chen X, Hu B (2020) Increased multidrug resistant isolates: new clinical burdens for 66 hospitals in Shanghai, 2015 to 2017. J Transl Med 8(4):112. https://doi.org/10.21037/atm.2019.12.91
Madhumita M, Guha P, Nag A (2019) Extraction of betel leaves (Piper Betle L.) essential oil and its bio-actives identification: process optimization, GC-MS analysis and anti-microbial activity. Ind Crops Prod 138:111578. https://doi.org/10.1016/j.indcrop.2019.111578
Madhumita M, Guha P, Nag A (2020) Bio-actives of betel leaf (Piper Betle L.): A comprehensive review on extraction, isolation, characterization, and biological activity. Phytother Res 34(10):2609–2627. https://doi.org/10.1002/ptr.6715
Magiorakos AP, Srinivasan A, Carey RB, Carmeli Y, Falagas ME, Giske CG, Harbarth S, Hindler JF, Kahlmeter G, Olsson-Liljequist B, Paterson DL (2012) Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect 18:268–281. https://doi.org/10.1111/j.1469-0691.2011.03570.x
Miller SI (2016) Antibiotic resistance and regulation of the gram-negative bacterial outer membrane barrier by host innate immune molecules. MBio 7(5):10–128
Ojha S, Raj A, Roy A, Roy S (2018) Extraction of total phenolics, flavonoids and tannins from Paederia foetida L. Leaves and their relation with antioxidant activity. Pharmacogn J 10:541–547. https://doi.org/10.5530/pj.2018.3.88
Oliveira SDDS, De Oliveira E, Silva AM, Blank AF, Nogueira PCLD et al (2020) Radical scavenging activity of the essential oils from Croton grewioides Baill accessions and the major compounds Eugenol, Methyl Eugenol and Methyl chavicol. J Essent Oil Res 33(1):94–103. https://doi.org/10.1080/10412905.2020.1779139
Patra B, Sahu A, Meena R, Pradhan SN (2021) Estimation of genetic diversity in Piper betle L. based on the analysis of morphological and molecular markers. Lett Appl Nano BioScience 10:2240–2250. https://doi.org/10.33263/LIANBS102.22402250
Pisoschi AM, Pop A, Cimpeanu C, Predoi G (2016) Antioxidant capacity determination in plants and plant-derived products: A review. Oxid Med Cell Longev 2016:1–36. https://doi.org/10.1155/2016/9130976
Proestos C, Sereli D, Komaitis M (2006) Determination of phenolic compounds in aromatic plants by RP-HPLC and GC-MS. Food Chem 95:44–52. https://doi.org/10.1016/j.foodchem.2004.12.016
Raj A, Sikdar B, Roy A, Mukhopadhyay AK, Roy S (2021) Antioxidant and antibacterial activities of phytochemicals in methanolic extracts of five underutilized leafy vegetables. Res J Biotech 16:1–10. https://doi.org/10.25303/168rjbt0121
Rao AV, Balachandran B (2002) Role of oxidative stress and antioxidants in neurodegenerative diseases. Nutr Neurosci 5(5):291–309
Rawat AKS, Tripathi RD, Khan AJ, Balasubrahmanyam VR (1989) Essential oil components as markers for identification of Piper betle L. cultivars. Biochem Syst Ecol 17:35–38. https://doi.org/10.1016/0305-1978(89)90039-2
Reller LB, Weinstein M, Jorgensen JH, Ferraro MJ (2009) Antimicrobial susceptibility testing: a review of general principles and contemporary practices. Clin Infect Dis 49:1749–1755. https://doi.org/10.1086/647952
Rice-Evans C, Miller N, Paganga G (1997) Antioxidant properties of phenolic compounds. Trends Plant Sci 2(4):152–159
Roleira FMF, Tavares-da-Silva EJ, Varela CL, Costa SC, Silva T, Garrido J, Borges F (2015) Plant derived and dietary phenolic antioxidants: anticancer properties. Food Chem 183:235–258
Sarma C, Rasane P, Kaur S, Singh J, Singh J, Gat Y, Garba U, Kaur D, Dhawan K (2018) Antioxidant and antimicrobial potential of selected varieties of Piper betle L. (Betel leaf). Acad Bras Cienc 90:3871–3878
Sikdar B, Raj A, Roy S (2022) Exploration of antibacterial and antioxidant potential of a few members of the family Piperaceae. Res J Biotech 17:59–69. https://doi.org/10.25303/281rjce1210132
Sleight SC, Wigginton NS, Lenski RE (2006) Increased susceptibility to repeated freeze-thaw cycles in Escherichia coli following long-term evolution in a benign environment. BMC Evol Biol 6(1):1–8
Syahidah A, Saad CR, Hassan MD, Rukayadi Y, Norazian MH, Kamarudin MS (2017) Phytochemical analysis, identification and quantification of antibacterial active compounds in betel leaves, Piper betle methanolic extract. Pak J Biol Sci 20:70–81. https://doi.org/10.3923/pjbs.2017.70.81
Terreni M, Taccani M, Pregnolato M (2021) New antibiotics for Multidrug-Resistant bacterial strains: latest research developments and future perspectives. Molecules 26:2671. https://doi.org/10.3390/molecules26092671
Torrijos R, Righetti L, Cirlini M, Calani L, Mañes J, Meca G, Dall’Asta C (2023) Phytochemical profiling of volatile and bioactive compounds in yellow mustard (Sinapis alba) and Oriental mustard (Brassica juncea) seed flour and Bran. LWT 173:114221. https://doi.org/10.1016/j.lwt.2022.114221
Valle DL, Cabrera EC, Puzon JJM, Rivera WL (2016) Antimicrobial activities of methanol, ethanol and supercritical CO2 extracts of Philippine Piper betle L. on clinical isolates of gram positive and gram negative Bacteria with transferable multiple drug resistance. PLoS ONE 11:e0146349. https://doi.org/10.1371/journal.pone.0146349
Zheng W, Wang SY (2001) Antioxidant activity and phenolic compounds in selected herbs. J Agric Food Chem 49:5165–5170. https://doi.org/10.1021/jf010697n
Author Information
Department of Botany, University of Kalyani, Kalyani, Nadia, West Bengal, India